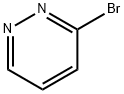
Pyridazine, 3-bromo- (9CI) synthesis
- Product Name:Pyridazine, 3-bromo- (9CI)
- CAS Number:88491-61-6
- Molecular formula:C4H3BrN2
- Molecular Weight:158.98
504-30-3
243 suppliers
$6.00/1g

88491-61-6
134 suppliers
$11.00/250mg
Yield:88491-61-6 69%
Reaction Conditions:
with phosphorus(V) oxybromide at 80 - 120; for 3 h;
Steps:
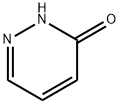
Synthesis of 3-bromopyridazine
Synthesis of 3-bromopyridazinePhosphorous oxybromide (158 g, 552 mmol) was heated to 80 °C under mechanical stirring until molten.3-Hydroxypyridazine (30.5 g, 317 mmol) was added in one portion to the orange liquid, which affordedimmediately a yellow, then a black solid. This was heated to 120 °C and left at this temperature for 3 h. After cooling the black solid was cooled with an ice water bath, small portions of ice water (in total; 300 ml) were slowly added and an exotherm was observed (white smoke). During stirring, some solids didn’t dissolve 2 M aqueous NaOH (180 mL) was added at RT and stirred for 45 mm until all solids weredissolved. The dark red/brown solution was poured into a mechanically stirred ice/water bath, which contained 2 M aqueous NaOH solution (910 mL). The internal temperature was kept below 25 “C during the addition. The pH was adjusted to -9.5 by addition of 2 M aqueous NaOH (50 mL). The brown solution was extracted with DCM (5x 250 mL). The combined yellow organic layer was dried over Na2SO4, filtered and concentrated in vacuo to obtain a grey/brown solid. The crude material was coated on silica (98 g)and filtered with heptane/EtOAc (1:1) over a plug of silica (200 g). Product containing fractions were combined and concentrated in vacuo to afford 3-bromopyridazine (34.7 g 216 mmol, 69%) as a green/grey solid. GCMS: >99% pure.
References:
GRüNENTHAL GMBH;NARDI, Antonio;RATCLIFFE, Paul;CRAAN, Tobias;HERTRAMPF, Thorsten;LESCH, Bernhard;KIME, Robert;STEINHAGEN, Henning WO2015/161928, 2015, A1 Location in patent:Page/Page column 68

504-30-3
243 suppliers
$6.00/1g

88491-61-6
134 suppliers
$11.00/250mg