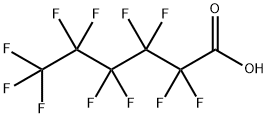
Perfluorohexanoic acid synthesis
- Product Name:Perfluorohexanoic acid
- CAS Number:307-24-4
- Molecular formula:C6HF11O2
- Molecular Weight:314.05
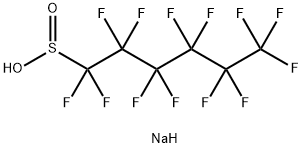
102061-83-6
1 suppliers
inquiry

307-24-4
202 suppliers
$20.00/2g
Yield:307-24-4 75%
Reaction Conditions:
with 2,2'-azobis(2-methylpropionamidine) dihydrochloride;water at 55; for 6.5 h;
Steps:
4
Example 4: Conversion of the sulphinate [5] to the carboxylic acid
References:
WO2005/121060,2005,A1 Location in patent:Page/Page column 6-7
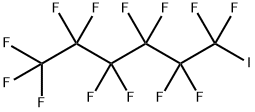
355-43-1
248 suppliers
$10.00/10g

307-24-4
202 suppliers
$20.00/2g

102061-83-6
1 suppliers
inquiry
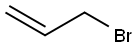
106-95-6
433 suppliers
$10.00/5g

307-24-4
202 suppliers
$20.00/2g
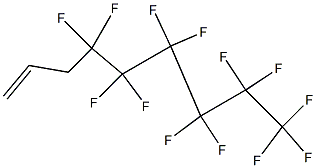
80793-18-6
3 suppliers
inquiry

102061-83-6
1 suppliers
inquiry
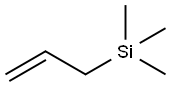
762-72-1
342 suppliers
$10.00/1g

307-24-4
202 suppliers
$20.00/2g
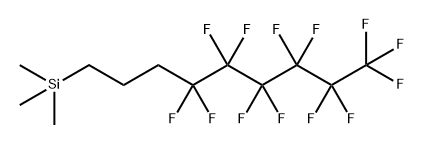
132673-95-1
0 suppliers
inquiry
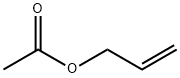
591-87-7
196 suppliers
$10.00/10g

102061-83-6
1 suppliers
inquiry

307-24-4
202 suppliers
$20.00/2g
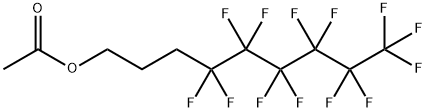
83311-03-9
0 suppliers
inquiry