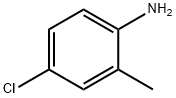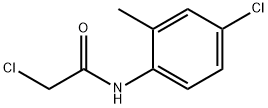
N1-(4-CHLORO-2-METHYLPHENYL)-2-CHLOROACETAMIDE synthesis
- Product Name:N1-(4-CHLORO-2-METHYLPHENYL)-2-CHLOROACETAMIDE
- CAS Number:62593-77-5
- Molecular formula:C9H9Cl2NO
- Molecular Weight:218.08
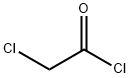
79-04-9
375 suppliers
$12.00/5g
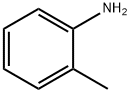
95-53-4
452 suppliers
$10.00/1g

62593-77-5
32 suppliers
$28.60/250MG
Yield:62593-77-5 842 g
Reaction Conditions:
Stage #1: chloroacetyl chloride;o-toluidinewith triethylamine in dichloromethane at 0 - 30; for 4 h;
Stage #2: with sulfuryl dichloride in 1,2-dichloro-ethane at 0 - 25; for 16 h;
Steps:
6 Example-6: In-situ- synthesis of 2-chloro-N-(4-chloro-2-methylphenyl)acetamide from o-toluidine
To a solution of o-toluidine (590 g, 4573 mmol) in dichlorethane (DCE) (2400 mL), triethyl amine (532 mL, 5259 mmol) was added at 25 °C. The mixture was cooled to 0°C and a solution of 2- chloroacetyl chloride (614 mL, 5259 mmol) in dichlorethane (DCE)(400 mL) was slowly added at 0- 10 °C. The reaction mixture was stirred for 4 h at 25-30 °C. After complete consumption of o- toluidine, the reaction mixture was cooled to 0 °C and a solution of sulfuryl chloride (715 g, 5030 mmol) in DCE (400 mL) was added during 4 h and stirred at 25 °C for 12 h. After completion of the reaction, excess solvent was distilled at reduced pressure and water (2000 mL) was added to it. The resulting precipitated product was filtered, washed with water (500 mL X 2) and dried under reduced pressure to obtain 2-chloro-N-(4-chloro-2-methylphenyl)acetamide (842 g, 3864 mmol).
References:
WO2022/64454,2022,A1 Location in patent:Page/Page column 31; 32
37394-93-7
75 suppliers
$28.60/25MG

62593-77-5
32 suppliers
$28.60/250MG

95-53-4
452 suppliers
$10.00/1g

62593-77-5
32 suppliers
$28.60/250MG