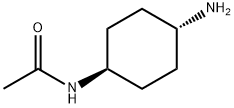
N-(trans-4-aMinocyclohexyl)-AcetaMide synthesis
- Product Name:N-(trans-4-aMinocyclohexyl)-AcetaMide
- CAS Number:873537-23-6
- Molecular formula:C8H16N2O
- Molecular Weight:156.23
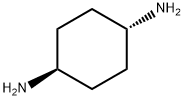
2615-25-0
272 suppliers
$5.00/1g
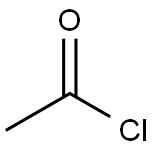
75-36-5
582 suppliers
$17.92/100G

873537-23-6
12 suppliers
$167.00/500MG
Yield:873537-23-6 94.7%
Reaction Conditions:
with pyridine in dichloromethane at 20; for 6 h;
Steps:
1.1; 2.1; 3.1 Step 1:
Add 100g trans-1,4-cyclohexanediamine and 103.91g pyridine into 500mL dichloromethane, add 69.44g acetyl chloride dropwise to the system at 0°C, and then react at room temperature for 6h. After the solvent was spin-dried, dissolved in ethyl acetate, and washed with weak acid conditions; the organic phase was dried, evaporated, and purified to obtain 128.61 g of intermediate 1 with a yield of 94.7%
References:
CN114835610,2022,A Location in patent:Paragraph 0071-0076

2615-25-0
272 suppliers
$5.00/1g
108-24-7
5 suppliers
$14.00/250ML

873537-23-6
12 suppliers
$167.00/500MG