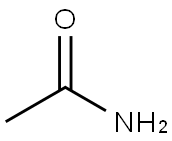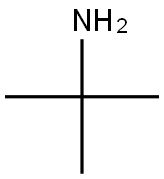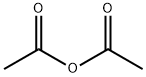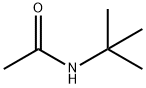
N-TERT-BUTYLACETAMIDE synthesis
- Product Name:N-TERT-BUTYLACETAMIDE
- CAS Number:762-84-5
- Molecular formula:C6H13NO
- Molecular Weight:115.17
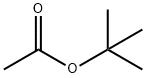
540-88-5
383 suppliers
$21.00/25mL
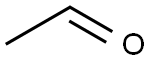
75-07-0
425 suppliers
$14.00/5mL

762-84-5
44 suppliers
$60.39/10g
Yield: 85%
Reaction Conditions:
Stage #1:acetaldehyde with sodium azide;trifluorormethanesulfonic acid;acetic acid at 40; for 2 h;Schmidt Reaction;
Stage #2:acetic acid tert-butyl ester with trifluorormethanesulfonic acid at 40;Ritter Amidation;
Steps:
General procedure for the syntheses of Substituted N-(tert-butyl)benzamides
General procedure: To a magnetically stirred solution of benzaldehyde (1 mmol) and NaN3 (1.5 mmol) in AcOH (0.2 mL), TfOH (1 mmol) was added and the reaction mixture was heated at 40 °C for 1-2 hrs. On the complete conversion of aldehyde (monitored by TLC) tert-butyl acetate was added alongwith TfOH (1 mmol). The reaction was continued to heating at 40 °C for another 3-5 hrs. After the completion, the reaction was brought to room temperature and ice-cooled aq. sat. NaHCO3 was added to the reaction mixture. The precipitate formed was filtered and washed with plenty of cold water and dried to obtain the desired benzamide as white solid in 96% yield. (Compounds obtained were pure hence, no further purification or recrystallisation was required).
References:
Singh, Garima;Dada, Ravikrishna;Yaragorla, Srinivasarao [Tetrahedron Letters,2016,vol. 57,# 39,p. 4424 - 4427] Location in patent:supporting information

540-88-5
383 suppliers
$21.00/25mL

75-05-8
1016 suppliers
$10.00/10g

762-84-5
44 suppliers
$60.39/10g

75-05-8
1016 suppliers
$10.00/10g
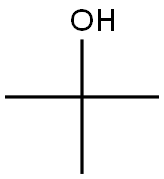
75-65-0
770 suppliers
$10.00/10ml

762-84-5
44 suppliers
$60.39/10g