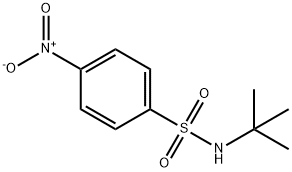
N-tert-Butyl 4-Nitrophenylsulfonamide synthesis
- Product Name:N-tert-Butyl 4-Nitrophenylsulfonamide
- CAS Number:49690-09-7
- Molecular formula:C10H14N2O4S
- Molecular Weight:258.29
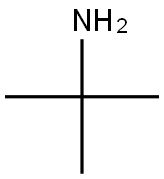
75-64-9
443 suppliers
$10.00/10g
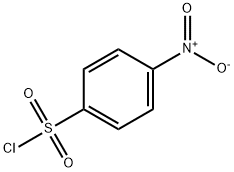
98-74-8
171 suppliers
$10.00/5g

49690-09-7
43 suppliers
$45.00/10mg
Yield:49690-09-7 97%
Reaction Conditions:
Stage #1: tert-butylamine;4-Nitrobenzenesulfonyl chloride in tetrahydrofuran at 20; for 24 h;
Stage #2: with hydrogenchloride in chloroform;water;Acidic aqueous solution;
Steps:
1.A.a EXAMPLE 14-Amino-N-tert-butylbenzenesulfonamideMethod A: a) N-tert-Butyl-4-nitrobenzenesulfonamide
a) N-tert-Butyl-4-nitrobenzenesulfonamide To a solution of tert-butylamine (0.47 L, 6.4 mol) in THF (0.55 L) is slowly added, at 0 °C, a solution of 4-nitrobenzenesulfonyl chloride (50 g, 0.23 mol) in THF (0.55 L) and the resulting mixture is stirred for 24 h at room temperature.. The solvent is removed and the residue is taken up in a CHCl3/0.5 N HCl mixture, the layers are separated and the aqueous phase is extracted with CHCl3.. The combined organic extracts are washed with H2O and brine and dried over MgSO4.. The solvent is removed, yielding 56.3 g of a yellowish solid which is directly used in the next reaction (yield: 97%). Mp: 105-109°C; 1H-NMR (300 MHz, CDCl3) δ (TMS): 1.29 (s, 9 H), 5.07 (s, 1 H), 8.13 (d, J = 9 Hz, 2 H), 8.39 (d, J = 9 Hz, 2 H).
References:
EP1424329,2004,A1 Location in patent:Page 8
2216-51-5
704 suppliers
$5.00/100mg
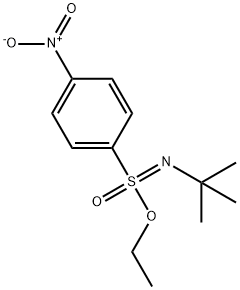
1569262-64-1
0 suppliers
inquiry

49690-09-7
43 suppliers
$45.00/10mg
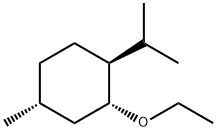
13947-22-3
0 suppliers
inquiry
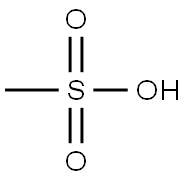
75-75-2
652 suppliers
$10.00/5g

1569262-64-1
0 suppliers
inquiry

49690-09-7
43 suppliers
$45.00/10mg
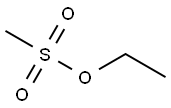
62-50-0
303 suppliers
$11.00/1g
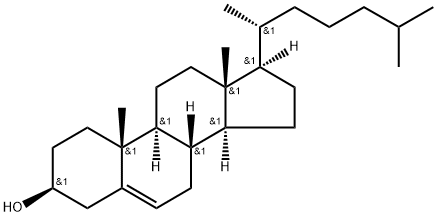
57-88-5
836 suppliers
$10.00/1g

1569262-64-1
0 suppliers
inquiry

49690-09-7
43 suppliers
$45.00/10mg
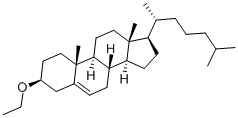
986-19-6
16 suppliers
inquiry
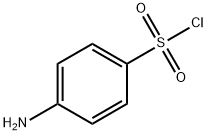
24939-24-0
40 suppliers
inquiry

75-64-9
443 suppliers
$10.00/10g

49690-09-7
43 suppliers
$45.00/10mg