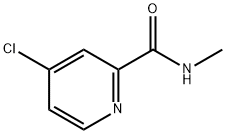
4-Chloro-N-methylpicolinamide synthesis
- Product Name:4-Chloro-N-methylpicolinamide
- CAS Number:220000-87-3
- Molecular formula:C7H7ClN2O
- Molecular Weight:170.6
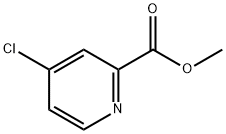
24484-93-3
408 suppliers
$6.00/5g
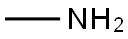
74-89-5
7 suppliers
$13.44/25ML

220000-87-3
484 suppliers
$8.00/5g
Yield:220000-87-3 98.5%
Reaction Conditions:
with magnesium chloride in tetrahydrofuran at 20; for 2.25 h;
Steps:
13 g (62.5 MMOL) 10 are dissolved together with 2.98 g (31.24 MMOL) dry MAGNESIUMCHLORIDE in THF. After 5 min 110 ML methyl amine solution (2M in THF) are added dropwise within 10 min and the suspension stirred for 2 h at room temperature. 120 ML water und 63 mL 1M HCI-SOLUTION are added and the mixture is extracted three times with ethyl acetate. The combined organic phases are washed with brine, dried with NA2SO4, filtered and evaporated. Yield : 10.5 g (98.5 %) 11, colourless oil. 13 g (62.5 MMOL) of compound 17 are dissolved in THF together with 2.98 g (31.24 MMOL) dry MGCI2. After five minutes, 110 ML methyl amine- solution (2M in THF) are added within ten minutes and the resulting suspension stirred for two hours at room temperature. The reaction mixture is treated with 120 mi water and 63 ml 1N hydrochloric acid and extracted three times with ethyl acetate. The combined organic phases are washed with brine, dried over NA2SO4, filtered and evaporated. Yield : 10.5 g (98.5 %) of compound 18, colourless oil.
References:
MERCK PATENT GMBH WO2004/37789, 2004, A2 Location in patent:Page 303-304; 315-316
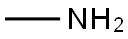
74-89-5
7 suppliers
$13.44/25ML

220000-87-3
484 suppliers
$8.00/5g
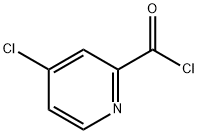
53750-66-6
101 suppliers
$60.00/250 mg
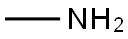
74-89-5
7 suppliers
$13.44/25ML

220000-87-3
484 suppliers
$8.00/5g
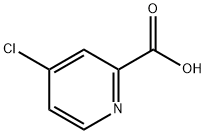
5470-22-4
315 suppliers
$7.00/5g
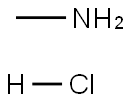
593-51-1
546 suppliers
$21.00/100g

220000-87-3
484 suppliers
$8.00/5g