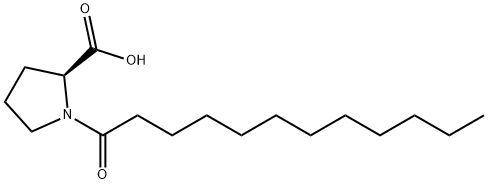
N-Dodecanoyl-L-proline synthesis
- Product Name:N-Dodecanoyl-L-proline
- CAS Number:58725-39-6
- Molecular formula:C17H31NO3
- Molecular Weight:297.43
Yield:58725-39-6 70%
Reaction Conditions:
Stage #1: lauric acidwith 1-hydroxy-pyrrolidine-2,5-dione;dicyclohexyl-carbodiimide in tetrahydrofuran at 20; for 24 h;
Stage #2: L-prolinewith N-ethyl-N,N-diisopropylamine in tetrahydrofuran;water at 20; for 24 h;
Steps:
A1 Product Obtained by Coupling Between Lauric Acid and L-Proline
To a solution of dodecanoc acid (19.77 g, 98.70 mmol) in THF (1 L) are successively added dicyclohexyl carbodiimide (DCC) (20.77 g, 100.68 mmol) and N-hydroxysuccinimide (NHS) (11.59 g, 100.68 mmol). After stirring for 24 h at room temperature, the medium is cooled down to 0° C. and filtered on frit. L-proline (12.50 g, 108.57 mmol), diisopropylethylamine (DIPEA) (63.78 g, 493.51 mmol) and water (90 mL) are added to the filtrate. After stirring for 24 h to room temperature, the medium is concentrated under reduced pressure, then dissolved into water (300 mL). The aqueous phase is washed with ethyl acetate (2×400 mL), acidified until pH 1 with a 1 N HCl aqueous solution then extracted with dichloromethane (3×250 mL). The combined organic phases are dried over Na2SO4, filtered off, and concentrated under reduced pressure. Aprs purification by chromatography on silica gel (cyclohexane, ethyl acetate), a colourless oil of molecule 1 is obtained. Yield: 20.53 g (70%) NMR 1H (CDCl3, ppm): 0.87 (3H); 1.26 (16H); 1.70 (2H); 1.90-2.10 (3H); 2.35 (2H); 2.49 (1H); 3.48 (1H); 3.56 (1H); 4.60 (1H) LC/MS (ESI): 298.2; (calcul ([M+H]+): 298.3)
References:
US2020/405819,2020,A1 Location in patent:Paragraph 0497-0499
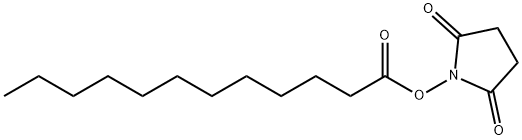
14565-47-0
55 suppliers
$22.00/250mg
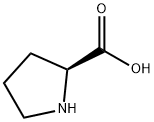
147-85-3
1033 suppliers
$6.00/25g

58725-39-6
56 suppliers
inquiry
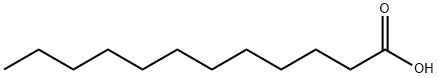
143-07-7
765 suppliers
$5.00/25g

58725-39-6
56 suppliers
inquiry