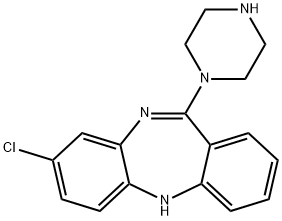
N-DESMETHYLCLOZAPINE synthesis
- Product Name:N-DESMETHYLCLOZAPINE
- CAS Number:6104-71-8
- Molecular formula:C17H17ClN4
- Molecular Weight:312.8
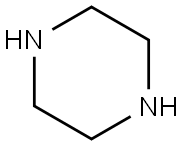
110-85-0
563 suppliers
$5.00/5 g
![8-Chloro-5,10-dihydrodibenzo[b,e][1,4]diazepin-11-one](/CAS/GIF/50892-62-1.gif)
50892-62-1
181 suppliers
$50.00/1 g

6104-71-8
130 suppliers
$74.00/5mg
Yield:6104-71-8 33%
Reaction Conditions:
with titanium tetrachloride in methoxybenzene at 20 - 125; for 5 h;
Steps:
A1
Example A1; Coupling of Piperazine A; 100 L enamelled reactor is charged with anisole (16 L) at 20° C. inner temperature and TiCl4 (1.064 kg, 1.37 equivalents) is added. The feed tank is rinsed with anisole (210 mL). Piperazine (2.113 kg, 6 equivalents) are added and the resulting brown suspension is warmed to 55° C. inner temperature. No significant exothermic reaction is observed. The compound of formula II (1.001 kg, 1 equivalent) is added at 55-60° C. inner temperature in portions over 30 minutes. An exothermic reaction occurs after the addition of the first portion, and the inner temperature raises to 65° C. (external cooling at -5° C. is applied). After the addition is complete, the brown reaction mixture is heated to 125° C. jacket temperature (120-124° C. inner temperature) and stirred for 4.5 h at this temperature. In process control by HPLC shows a conversion of 99%; Filtration of Titanium Salts; The reaction mixture is cooled to -2° C. inner temperature. NaOH (30%, 2.4 L, 5.5 equivalents) is added at this temperature over 80 minutes (an exothermic reaction occurs). After the addition is complete, the resulting suspension is warmed to 22° C. inner temperature over 60 minutes. The titanium salts form a well filterable, granulated solid which is filtered off over a pad of celite (10 L pressure filter). The reactor and the filter cake are washed with t-butyl methyl ether (TBME, 10 L). The brown filtrate (29 L) is washed with NaOH (0.1 M, 7 L); Extractive Workup; The organic phase is extracted with HCl in three portions (1 M, 8+7+3.5 L). The acidic aqueous layers are combined and washed with TBME (4.5 L). TBME (6.5 L) is added to the aqueous phase and the pH is adjusted to 13 by the addition of NaOH (30%, 2.5 L). The organic layer is separated and the aqueous layer is extracted with TBME (6 L). The combined TBME-layers are washed with half-saturated brine in two portions (2×4 L), then filtered over a 10 L pressure filter charged with Na2SO4 (3.97 kg). The filter cake is washed with TBME in portions (9 L in total); Crystallization from TBME; The combined filtrates (approximately 25 L) are concentrated under reduced pressure (350 mbar, 45° C. jacket temperature) to a residual volume of approximately 1.5 L. The residual brown thick solution is warmed to 40° C. inner temperature, then cooled to -1° C. A thick yellow suspension is formed, which is diluted with TBME (2 L). Stirring at this temperature is continued for approximately 60 minutes. The suspension is filtered off (10 L pressure filter, 1200 mbar). The solids are dried on a rotary evaporator under reduced pressure at 80° C. for approximately 7 h. The operation yields 542.11 g of a yellow solid, containing approximately 3.75 percent by weight of TBME as determined by NMR; Water Slurry and Final Drying; The yellow solid is suspended in water (5.5 L) and the mixture is stirred 20 hours at 22° C. inner temperature. The solid is filtered off(10 L pressure filter, 1200 mbar). The filter cake is rinsed with water in portions (in total 4 L). The product is dried for 3 days on the filter in a stream of nitrogen, and then further dried under reduced pressure (<20 mbar), at 60° C. bath temperature for 5 hours to yield 427.71 g of N-desmethylclozapine as a yellow solid (33% based on the amount of 8-chloro-11-oxo-10,11-dihydro-5H-dibenzo-1,4-diazepin). The melting range of the product is 110.6-124.1° C. and the solid product is a non-crystalline and amorphous product, as shown by powder X-ray diffraction measurement.
References:
Tolf, Bo-Ragnar;Thygesen, Mikkel Boas;Blatter, Fritz;Berghausen, J?rg US2005/282800, 2005, A1 Location in patent:Page/Page column 14
5786-21-0
467 suppliers
$14.00/100mg

6104-71-8
130 suppliers
$74.00/5mg
67990-66-3
60 suppliers
$38.00/250mg

6104-71-8
130 suppliers
$74.00/5mg
![8-Chloro-5,10-dihydrodibenzo[b,e][1,4]diazepin-11-one](/CAS/GIF/50892-62-1.gif)
50892-62-1
181 suppliers
$50.00/1 g

6104-71-8
130 suppliers
$74.00/5mg
![8,11-Dichloro-5H-dibenzo[b,e][1,4]diazepine](/CAS/GIF/50373-22-3.gif)