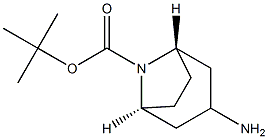
N-Boc-exo-3-aminotropane synthesis
- Product Name:N-Boc-exo-3-aminotropane
- CAS Number:744183-20-8
- Molecular formula:C12H22N2O2
- Molecular Weight:226.32
![tert-butyl 3-(benzylamino)-endo-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/20180601/GIF/207564-70-3.gif)
207564-70-3
1 suppliers
inquiry

744183-20-8
103 suppliers
$14.00/250mg
Yield:744183-20-8 94%
Reaction Conditions:
with 20% palladium hydroxide-activated charcoal;ammonium formate in ethanol at 50; for 3 h;Inert atmosphere;
Steps:
58.2 Step 2.
Step 2.
Preparation of tert-butyl (1R,3r,5S)-3-amino-8-azabicyclo[3.2.1]octane-8-carboxylate
To a mixture of 20% palladium hydroxide on carbon (4.93 g) in ethanol (200 mL) was added a solution of tert-butyl (1R,3r,5S)-3-(benzylamino)-8-azabicyclo[3.2.1]octane-8-carboxylate (49.3 g, 155.7 mmol) in ethanol (400 mL).
To the reaction mixture was added ammonium formate (49.3 g, 781.3 mmol) and the resulting mixture was sparged with nitrogen for 10 minutes.
The reaction mixture was heated to 50° C. for 3 h, allowed to cool to ambient temperature, and filtered through a bed of celite.
The filtrate was concentrated in vacuo to afford the title compound as a colorless solid (33.0 g, 94% yield): 1H NMR (300 MHz, CDCl3) δ 4.11 (d, J=23.1 Hz, 2H), 3.26 (t, J=6.2 Hz, 1H), 2.10-1.84 (m, 6H), 1.40 (s, 9H), 1.22 (s, 2H), NH not observed; MS (ES+) m/z 227.1 (M+1).
References:
Xenon Pharmaceuticals Inc.;Andrez, Jean-Christophe;Burford, Kristen Nicole;Dehnhardt, Christoph Martin;Focken, Thilo;Grimwood, Michael Edward;Jia, Qi;Lofstrand, Verner Alexander;Wesolowski, Steven Sigmund;Wilson, Michael Scott US2020/71313, 2020, A1 Location in patent:Paragraph 1057; 1060; 1061
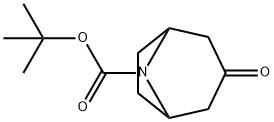
185099-67-6
244 suppliers
$5.00/250mg

744183-20-8
103 suppliers
$14.00/250mg
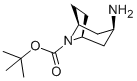
207405-68-3
110 suppliers
$16.00/100mg
![tert-butyl 3-endo-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/GIF/143557-91-9.gif)
143557-91-9
103 suppliers
$7.00/250mg

744183-20-8
103 suppliers
$14.00/250mg
![1,1-DiMethylethyl 3-[(phenylMethyl)aMino]-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/GIF/944123-76-6.gif)
944123-76-6
12 suppliers
$303.00/1 g

744183-20-8
103 suppliers
$14.00/250mg

185099-67-6
244 suppliers
$5.00/250mg

744183-20-8
103 suppliers
$14.00/250mg