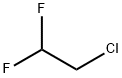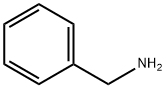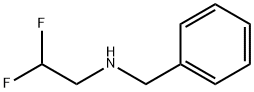
N-benzyl-N-(2,2-difluoroethyl)amine synthesis
- Product Name:N-benzyl-N-(2,2-difluoroethyl)amine
- CAS Number:1184224-96-1
- Molecular formula:C9H11F2N
- Molecular Weight:171.19
Yield:1184224-96-1 95.6%
Reaction Conditions:
at 120; for 16 h;Product distribution / selectivity;Autoclave;
Steps:
1.1
Example 1.1 An amount of 1152 g (11.1 mol) of 2,2-difluoro-1-chloroethane and 403 g of benzylamine (3.695 mol) are heated in an autoclave at an internal temperature of 120° C. for 16 hours. Subsequently, 700 g of water are added and the aqueous phase is separated. The aqueous phase comprises benzylamine hydrochloride, which is converted back into free benzylamine by addition of sodium hydroxide solution. The organic phase is first distilled at standard pressure, the unreacted 2,2-difluoro-1-chloroethane being distilled off. Vacuum distillation is then again carried out (at approximately 200 mbar), the remaining traces of 2,2-difluoro-1-chloroethane being distilled off. An amount of 306 g of N-benzyl-2,2-difluoroethanamine is obtained as distillation residue with a purity of 98.9%. This corresponds to a yield of 95.6%, based on the benzylamine reacted. It is possible, by renewed distillation, to obtain N-benzyl-2,2-difluoroethanamine in a purity of greater than 99%. 1H NMR (CDCl3): 7.24-7.35 (m, 5H), 5.84 (tt, 1H), 3.84 (s, 2H), 2.95 (dt, 2H)
References:
US2012/123163,2012,A1 Location in patent:Page/Page column 6