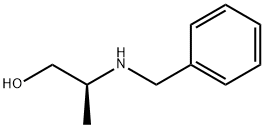
N-BENZYL-L-ALANINOL synthesis
- Product Name:N-BENZYL-L-ALANINOL
- CAS Number:6940-80-3
- Molecular formula:C10H15NO
- Molecular Weight:165.23
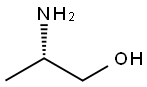
2749-11-3
408 suppliers
$14.56/5G
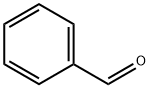
100-52-7
954 suppliers
$17.30/2g

6940-80-3
73 suppliers
$45.00/50mg
Yield: 86%
Reaction Conditions:
Stage #1:(S)-Alaninol;benzaldehyde in ethanol for 6 h;Reflux;
Stage #2: with sodium tetrahydroborate in ethanol at 20; for 4 h;Cooling with ice;
Steps:
General procedure A for the synthesis of 2-benzylamino alkyl alcohols (1a?1d, 1h?1j,1l)
General procedure: To a 200 mL round bottom flask, benzaldehyde (1.06 g, 10 mmol) and 2-aminalkylalcohol (10 mmol) and 50 mL of EtOH were added. The reaction system was then heatedup to reflux 6 h. After cooling the reaction to room temperature, NaBH4 (1.13 g, 30 mmol)was added stepwise for 1 h under ice-water bath. The reaction was then warmed up toroom temperature for 4 h. Reaction quenched with water (40 mL). The reaction mixturewas extracted with DCM (330 mL). The organic layer was collected, washed with brine(330 mL), and dried over with anhydrous sodium sulfate. After removing the solvent invacuo, 2-benzylamino alkyl alcohols 1 was purified through column chromatography with hexanes/EtOAc (20: 1 – 5:1) as eluent.
References:
Chen, Ning;Li, Xiang;Xu, Jiaxi [Synthesis,2019,vol. 51,# 17,p. 3336 - 3344] Location in patent:supporting information
2198-64-3
101 suppliers
$14.00/5G

6940-80-3
73 suppliers
$45.00/50mg
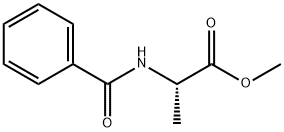
7244-67-9
46 suppliers
$25.00/1G

6940-80-3
73 suppliers
$45.00/50mg
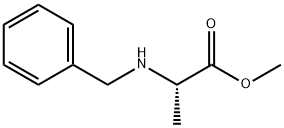
31022-10-3
29 suppliers
inquiry

6940-80-3
73 suppliers
$45.00/50mg

100-52-7
954 suppliers
$17.30/2g

6940-80-3
73 suppliers
$45.00/50mg