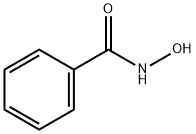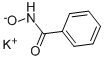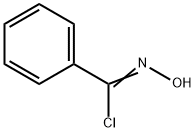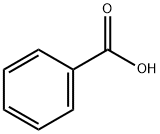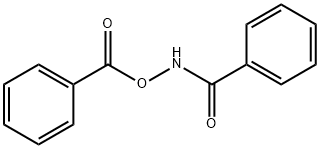
N-(benzoyloxy)benzamide synthesis
- Product Name:N-(benzoyloxy)benzamide
- CAS Number:959-32-0
- Molecular formula:C14H11NO3
- Molecular Weight:241.24
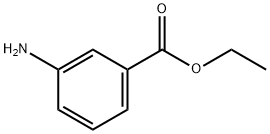
582-33-2
199 suppliers
$27.00/5g
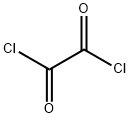
79-37-8
466 suppliers
$17.67/10gm:
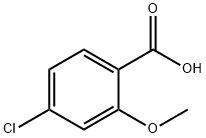
57479-70-6
168 suppliers
$10.00/1g

959-32-0
4 suppliers
inquiry
Yield:959-32-0 97%
Reaction Conditions:
with triethylamine in dichloromethane;N,N-dimethyl-formamide;
Steps:
1.a Example 1
(a) Ethyl 3-{(4-chloro-2-methoxybenzoyl)amino}benzoate (4: R1A=OMe, R2 and R4=H, R3A=Cl, R5=H and RA=Et) A suspension of 4-chloro-2-methoxybenzoic acid (10.0 g, 53.6 mmol), oxalyl chloride (8.84 g, 69.7 mmol) and DMF (50 μL) in CH2Cl2 (107 mL) was stirred at 25° for 2 h. The resulting clear solution was concentrated under reduced pressure. To the residual, crude acyl chloride in CH2Cl2 (107 mL) were added ethyl 3aminobenzoate (8.85 g, 53.6 mmol) and Et3N (10.8 g, 107 mmol) over 5 min at 25°. The mixture was stirred at 25° for 24 h. The resulting suspension was diluted with EtOAc (350 mL) and the resulting solution was successively washed with aqueous 1 N HCl (2*150 mL), aqueous saturated NaHCO3 (2*150 mL) and brine (150 mL). The organic layer was dried (MgSO4), filtered and concentrated under reduced pressure to give the desired benzoylaminobenzoate of formula 4 (17.4 g, 97% yield) as a beige solid: 1H NMR (DMSO-d6) δ 810.31 (s, 1H), 8.40 (broad s, 1H), 7.94 (broad d, J=7.9 Hz, 1H), 7.69 (dt, J=7.9, 1.2 Hz, 1H), 7.62 (d, J=8.2 Hz, 1H), 7.49 (t, J=7.9 Hz, 1H), 7.28 (d, J=1.9 Hz, 1H), 7.13 (dd, J=8.2, 1.9 Hz, 1H), 4.33 (q, J=7.0 Hz, 2H), 3.92 (s, 3H), 1.33 (t, J=7.0 Hz, 3H).
References:
US6323202,2001,B1
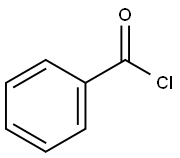
98-88-4
578 suppliers
$10.00/5g

959-32-0
4 suppliers
inquiry