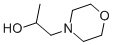
N-(2-HYDROXYPROPYL)MORPHOLINE synthesis
- Product Name:N-(2-HYDROXYPROPYL)MORPHOLINE
- CAS Number:2109-66-2
- Molecular formula:C7H15NO2
- Molecular Weight:145.2
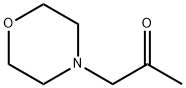
6704-35-4
29 suppliers
$65.00/50mg
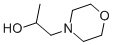
2109-66-2
93 suppliers
$17.67/250mgs:
Yield:-
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0 - 20; for 12 h;
Steps:
6.1 1-morpholinopropan-2-ol
1-morpholinopropan-2-ol (0630) [284] To a solution of l-morpholinopropan-2-one (0.4 g, 2.79 mmol) in MeOH (5 mL) at 0 °C was added NaBH4 (0.159 g, 4.19 mmol). The resulting reaction mixture was stirred at room temperature for 12 h. Progress of the reaction was monitored by TLC. After TLC indicated the reaction was complete, the reaction mixture was concentrated to dryness under reduced pressure. The residue was diluted with water and extracted with DCM. The combined organic layer was dried over anhydrous Na2S04, filtered, and concentrated to get a crude residue. The crude compound was purified by silica gel column chromatography eluting with 0-5% MeOH/DCM to afford the title compound as a yellow oil (0.18 g, yield 43.8%). 1H NMR (400 MHz, DMSO-d6) δ 4.26 (d, = 4.4 Hz, 1H), 3.78 - 3.72 (m, 1H), 3.55 (t, =4.8 Hz, 4H), 2.37 (t, =4.0 Hz, 4H), 2.25 - 2.11 (m, 2H), 1.02 (d, = 6.0 Hz, 3H).
References:
SYROS PHARMACEUTICALS, INC.;ROBERTS, Christopher;ZHANG, Yi;BEAUMIER, Francis;LEPISSIER, Luce;MARINEAU, Jason, J.;RAHL, Peter, B.;SPROTT, Kevin;CIBLAT, Stephen;SOW, Boubacar;LAROUCHE-GAUTHER, Robin;BERSTLER, Lauren WO2016/196910, 2016, A1 Location in patent:Paragraph 283; 284
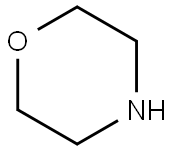
110-91-8
705 suppliers
$9.00/1g
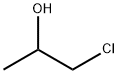
127-00-4
141 suppliers
$25.19/25g
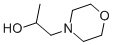
2109-66-2
93 suppliers
$17.67/250mgs:
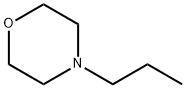
23949-50-0
7 suppliers
inquiry
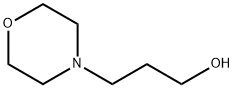
4441-30-9
146 suppliers
$14.00/5g
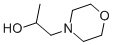
2109-66-2
93 suppliers
$17.67/250mgs:
696-57-1
54 suppliers
$45.00/50mg

4441-30-9
146 suppliers
$14.00/5g
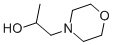
2109-66-2
93 suppliers
$17.67/250mgs: