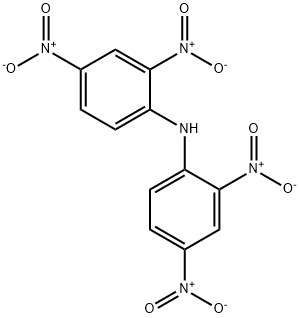
N-(2,4-dinitrophenyl)-2,4-dinitroaniline synthesis
- Product Name:N-(2,4-dinitrophenyl)-2,4-dinitroaniline
- CAS Number:2908-76-1
- Molecular formula:C12H7N5O8
- Molecular Weight:349.21

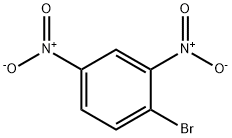
122-39-4
343 suppliers
$14.00/5g

2908-76-1
7 suppliers
inquiry
Yield:2908-76-1 81%
Reaction Conditions:
with bismuth (III) nitrate pentahydrate;palladium diacetate in 2,2,2-trifluoroethanol;trifluoroacetic acid at 20 - 90; for 24 h;Inert atmosphere;
Steps:
4.1. General procedures for dinitration of aromatic amines
General procedure: Bi(NO3)3.5H2O (2 equiv, 0.6 mmol), Pd(OAc)2 (0.015 mmol, 5 mol %) were added into a 25mL oven-dried Schlenk tube, the tube was evacuated and backfilled with Ar (repeated three times in 30 min). Under a counter flow of Ar, N-substituted aniline (1 equiv, 0.3 mmol) was added by syringe firstly, 1.5 mL TFE and 0.5 mL TFA were then added by disposable medical syringes. The flask was sealed and the mixture was stirred firstly at room temperature for15 min, then allowed to stir in a preheated oil bath at 90 °C for 24 h.The mixture was cooled to room temperature when the reaction was completed. Next, the solution was diluted by 10 mL ethyl acetate and filtered the insoluble matters, washed the organic phases by 5% NaHCO3 solution after the extraction, and extracted the aqueous phases by ethyl acetate (3x15 mL) to collect organic phases. All the organic solutions were washed by brine, dried 30 min with anhydrous MgSO4, filtered and concentrated under reduced pressure. The crude products were purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether(5/1-2/1).
References:
Feng, Yi-Si;Mao, Long;Bu, Xiao-Song;Dai, Jian-Jun;Xu, Hua-Jian [Tetrahedron,2015,vol. 71,# 23,p. 3827 - 3832]
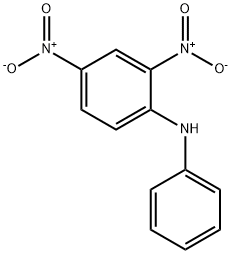
961-68-2
101 suppliers
$40.00/25g

2908-76-1
7 suppliers
inquiry
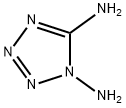
2165-21-1
15 suppliers
$140.00/1g
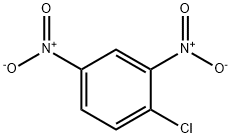
97-00-7
12 suppliers
$17.67/10gm:

2908-76-1
7 suppliers
inquiry
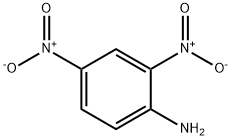
97-02-9
9 suppliers
$10.00/25g
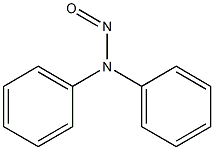
382165-80-2
2 suppliers
inquiry

2908-76-1
7 suppliers
inquiry