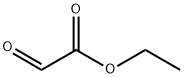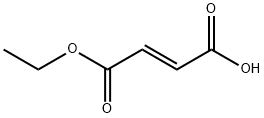
Monoethyl fumarate synthesis
- Product Name:Monoethyl fumarate
- CAS Number:2459-05-4
- Molecular formula:C6H8O4
- Molecular Weight:144.13
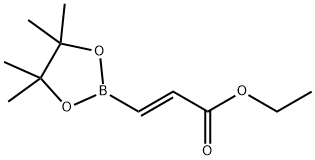
1009307-13-4
119 suppliers
$17.00/250mg

124-38-9
129 suppliers
$175.00/23402

2459-05-4
250 suppliers
$9.00/25g
Yield:2459-05-4 76%
Reaction Conditions:
with potassium methanolate;copper(l) chloride in N,N-dimethyl acetamide at 70; for 24 h;Sealed tube;Schlenk technique;
Steps:
13 Example 13
In the glove box, sequentially add trans-2-(ethoxycarbonyl)vinylboronic acid pinacol ester (1mmol, 226.1mg) to a 50mL Schlenk bottle equipped with a stir bar,Potassium methoxide (2mmol, 2 equivalents, 140.2mg), CuCl (0.03mmol, 0.03 equivalents, 3.0mg), 5mL solvent N,N-dimethylacetamide.Take out the capped Schlenk bottle from the glove box, fully ventilate, fill the reaction system with carbon dioxide and seal it, and then stir the reaction mixture at 70°C for 24 hours.After cooling to room temperature, add 1mol/L hydrochloric acid to acidify, and extract with ethyl acetate, and then wash with brine once.The organic phase was collected and concentrated in vacuo. The liquid mixture was dropped on a silica gel column and purified by column chromatography. The developing solvent was petroleum ether/ethyl acetate.The desired product trans-3-(ethoxycarbonyl)acrylic acid was obtained with a yield of 76percent.
References:
Peking University;Jiangsu Wei Ming Environmental Protection Technology Co., Ltd.;Hong Junting;Zhao Yu;Sun Yang;Mo Fanyang CN111217693, 2020, A Location in patent:Paragraph 0069-0070
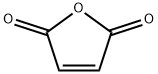
108-31-6
871 suppliers
$13.47/50 G

64-17-5
756 suppliers
$10.00/50g

2459-05-4
250 suppliers
$9.00/25g

124-38-9
129 suppliers
$175.00/23402
![3-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-acrylic acid ethyl ester](/CAS/GIF/1263187-14-9.gif)
1263187-14-9
33 suppliers
inquiry

2459-05-4
250 suppliers
$9.00/25g

108-31-6
871 suppliers
$13.47/50 G

2459-05-4
250 suppliers
$9.00/25g