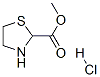
METHYL THIAZOLIDINE-2-CARBOXYLATE HYDROCHLORIDE synthesis
- Product Name:METHYL THIAZOLIDINE-2-CARBOXYLATE HYDROCHLORIDE
- CAS Number:50703-06-5
- Molecular formula:C5H10ClNO2S
- Molecular Weight:183.66

67-56-1
746 suppliers
$9.00/25ml
34592-47-7
394 suppliers
$5.00/5g

50703-06-5
38 suppliers
$45.00/5G
Yield:50703-06-5 99.3%
Reaction Conditions:
with thionyl chloride at 5; for 0.166667 h;Inert atmosphere;
Steps:
5.3.2 3-Nitroso-1,3-thiazolidine-4-carboxamide (4)
General procedure: Thionyl chloride (5.2 mL; 73.4 mmol) was dropped slowly into 20 mL distilled methanol in an ice bath below 5 °C under nitrogen gas; the reaction mixture was stirred for 10 min. l-Thioproline (2.7 g; 20.0 mmol) was added to the reaction mixture and stirred overnight. The organic solvent was evaporated three times after adding methanol, and the residue was washed twice with diethyl ether and decanted. This step was repeated. After evaporating the remaining solvent, methyl 1,3-thiazolidine-4-carboxylate hydrochloride was obtained (3.7 g; 99.3 % yield) as a white solid. 1H NMR (400 MHz, MeOH-d4) δ 4.81 (t, J=7.3Hz, 1H, N-CH-CO), 4.46 (d, J=9.2Hz, 1H, S-CH2-N), 4.40 (d, J=9.8Hz, 1H, S-CH2-N), 3.88 (s, 3H, COOCH3), 3.52 (dd, J=7.3, 12.2Hz, 1H, S-CH2-C), 3.41 (dd, J= 6.1, 12.2Hz, 1H, S-CH2-C).
References:
Inami, Keiko;Kondo, Sonoe;Ono, Yuta;Saso, Chiharu;Mochizuki, Masataka [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 24,p. 7853 - 7857]