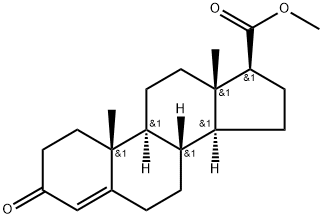
Methyl 3-oxo-4-androstene-17beta-carboxylate synthesis
- Product Name:Methyl 3-oxo-4-androstene-17beta-carboxylate
- CAS Number:2681-55-2
- Molecular formula:C21H30O3
- Molecular Weight:330.47
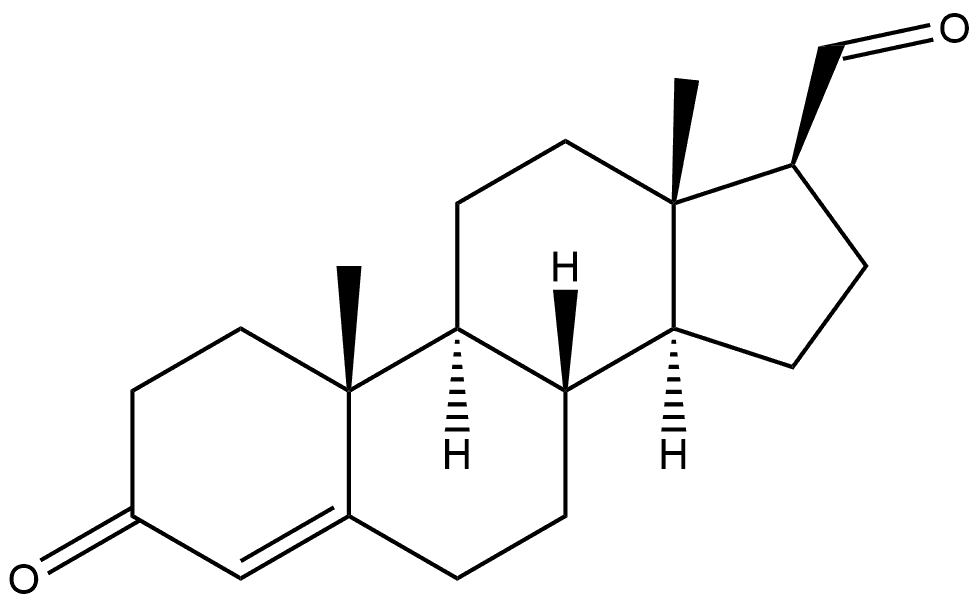
6247-91-2
0 suppliers
inquiry

2681-55-2
97 suppliers
inquiry
Yield:2681-55-2 93%
Reaction Conditions:
with tert.-butylhydroperoxide;potassium iodide in methanol at 20; for 6.5 h;Reflux;Reagent/catalyst;
Steps:
3; 6 Synthesis of androsten-4-en-3one-17β-carboxylic acid methyl ester (1)
To the reaction flask was added 30 g (0.1 mol) of androst-4-en-3one-17β-formaldehyde. 300 ml of anhydrous methanol and 1.66 g (10 mmol) of potassium iodide. Control the temperature at 20 ° C, dropwise addition of 27 g (0.3 mol) of t-butoxide peroxide, After about 30 minutes, the reaction was refluxed for 6 hours, and the reaction was completed by TLC. The reaction was terminated by adding 27.7 g (0.22 mol) of sodium sulfite (dissolved in 100 ml of water). Concentrate under reduced pressure and add 200 ml of water. Stir at room temperature for 2 hours, filter, Drying at 60 ° C gave 30.75 g (0.093 mol) of a white solid, yield: 93%.
References:
Hunan Yuxin Pharmaceutical Co., Ltd.;Liu Hong;Zhou Kailan;Shen Yuliang;Cao Chunyu;Wang Li;Peng Zhengzhong CN109467584, 2019, A Location in patent:Paragraph 0013; 0036; 0037; 0038; 0045; 0046; 0047
302-97-6
187 suppliers
$29.00/5mg
85622-93-1
534 suppliers
$5.00/50mg

2681-55-2
97 suppliers
inquiry
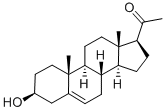
145-13-1
515 suppliers
$25.00/500mg

2681-55-2
97 suppliers
inquiry
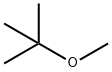
1634-04-4
688 suppliers
$14.00/100g

302-97-6
187 suppliers
$29.00/5mg

2681-55-2
97 suppliers
inquiry
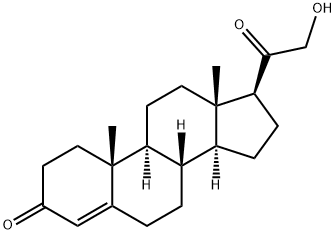
64-85-7
141 suppliers
$15.00/5mg

2681-55-2
97 suppliers
inquiry
