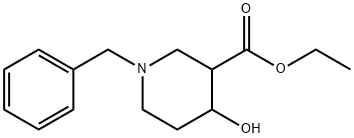
Methyl 1-benzyl-4-hydroxypiperidine-3-carboxylate synthesis
- Product Name:Methyl 1-benzyl-4-hydroxypiperidine-3-carboxylate
- CAS Number:956010-25-6
- Molecular formula:C15H21NO3
- Molecular Weight:263.33
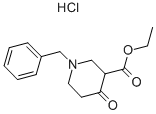
1454-53-1
247 suppliers
$5.00/5g

956010-25-6
38 suppliers
$45.00/100mg
Yield:956010-25-6 84%
Reaction Conditions:
Stage #1: ethyl 1-benzyl-4-oxopiperidine-3-carboxylate hydrochloridewith triethylamine in methanol at 20; for 0.166667 h;
Stage #2: with sodium tetrahydroborate in methanol at 20; for 3 h;
Stage #3: with hydrogenchloride;methanol;water;sodium hydrogencarbonatemore than 3 stages;
Steps:
48.a
(a) Racemic ethyl 4-hydroxy-l-(ρheny1methyl)-3-piperidinecarboxylate (mixture of cis and trans)To a solution of ethyl 4-oxo-l-(phenylmethyl)-3-piperidinecarboxylate hydrochloride (5Og, 170mmol) in methanol (11) was added triethylamine (28.3ml, 204mmol) and the mixture stirred at room temperature for lOmin under argon. Sodium borohydride (21.32g, 560mmol) was then added portionwise and the reaction stirred at room temperature for 3h. 5N HCl solution (175ml) was added (final pH=2-3) and the mixture reduced to approx. 200ml. The residue was neutralized with a saturated solution of sodium bicarbonate (150ml) and the aqueous phase was extracted with dichloromethane and then a 9:1 dichloromethane:methanol mixture.The organic layer was dried and the solvent was removed under reduced pressure. This provided the desired compound (37g, 84%) as a mixture ocis and trans isomers in an approximately 1 :1 ratio. MS (ES+) m/z 264 (MH+, 100%)
References:
WO2007/122258,2007,A1 Location in patent:Page/Page column 59
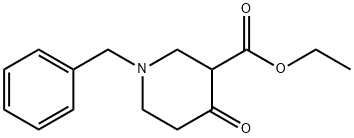
41276-30-6
104 suppliers
$61.96/1g

956010-25-6
38 suppliers
$45.00/100mg