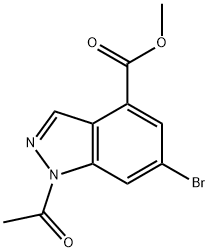
Methyl 1-Acetyl-6-broMo-1H-indazole-4-carboxylate synthesis
- Product Name:Methyl 1-Acetyl-6-broMo-1H-indazole-4-carboxylate
- CAS Number:1346597-55-4
- Molecular formula:C11H9BrN2O3
- Molecular Weight:297.1
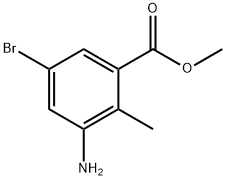
1000342-11-9
180 suppliers
$6.00/1g
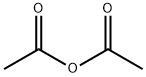
108-24-7
5 suppliers
$14.00/250ML

1346597-55-4
31 suppliers
$75.00/100mg
Yield:1346597-55-4 98.3%
Reaction Conditions:
Stage #1: 2-methyl-3-amino-5-bromobenzoic acid methyl ester;acetic anhydridewith potassium acetate in chloroform at 20; for 12 h;
Stage #2: with tert.-butylnitrite;18-crown-6 ether in chloroform at 65; for 3 h;
Steps:
[01042] Step 5: methyl l-acetyl-6-bromo-lH-indazole-4-carboxylate[01043] To a stirred solution of methyl 3-amino-5-bromo-2-methylbenzoate (15 g, 61.5 mmol) in chloroform (150 mL), was added potassium acetate (6.32 g, 64.4 mmol) and acetic anhydride (12.6 g, 122.9 mmol) and reaction mixture was stirred at room temperature for 12 h. After this time, tert-butyl nitrite (25.3g, 246.1 mmol) and 18-crown-6 (5.7 g, 21.5 mmol) were added and reaction stirred again at 65 °C for 3 h. On completion, the reaction mass was cooled to room temperature, diluted with chloroform (500 mL) and washed with sat. sodium bicarbonate solution. The organic layer was dried over sodium sulfate and concentrated to afford the title compound (18 g, 98.3%).
References:
WO2012/118812,2012,A2 Location in patent:Page/Page column 312-313
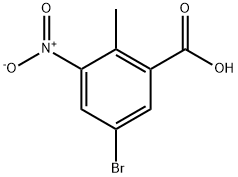
107650-20-4
157 suppliers
$9.00/250mg

1346597-55-4
31 suppliers
$75.00/100mg
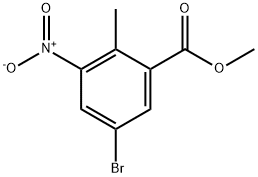
220514-28-3
149 suppliers
$10.00/1g

1346597-55-4
31 suppliers
$75.00/100mg
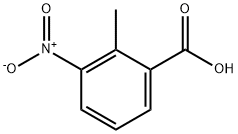
1975-50-4
449 suppliers
$20.00/25g

1346597-55-4
31 suppliers
$75.00/100mg