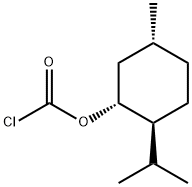
(-)-MENTHYL CHLOROFORMATE synthesis
- Product Name:(-)-MENTHYL CHLOROFORMATE
- CAS Number:14602-86-9
- Molecular formula:C11H19ClO2
- Molecular Weight:218.72
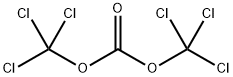
32315-10-9
432 suppliers
$10.00/1g
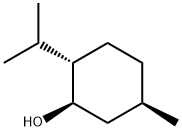
2216-51-5
704 suppliers
$5.00/100mg

14602-86-9
145 suppliers
$14.00/1g
Yield:14602-86-9 100%
Reaction Conditions:
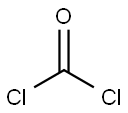
Stage #1:bis(trichloromethyl) carbonate with pyridine in toluene at 0;Inert atmosphere;
Stage #2:(-)-menthol in toluene at 0 - 20;Inert atmosphere;
Steps:
4.2 RRN 5Synthesis of (-)-menthyl carbazate 3
General procedure: 4.2 RRN 5Synthesis of (-)-menthyl carbazate 3 (0010) A solution of pyridine (21.9mL, 0.27mol) in toluene (150mL) was added dropwise to a stirred solution of triphosgene (21.96g, 0.074mol) in toluene (260mL) at 0°C under an argon atmosphere. Stirring was continued for 15min at 0°C and a solution of (-)-menthol 1 (28.12g, 0.18mol) in toluene (100mL) was then added slowly through a dropping funnel. The reaction mixture was allowed to warm to rt and stirred for 15h. The reaction mixture was then diluted with water (300mL) and extracted with toluene (2×200mL). The combined organic layers were washed sequentially with water (200mL) and brine (200mL) and dried (Na2SO4). The solvent was removed in vacuo to give (-)-menthyl chloroformate 2 as a colorless oil (39.3g), which was used directly in the next step without further purification. (0011) To a stirred solution of benzyltriethylammonium chloride (0.02g, 0.1mmol) and K2CO3 (2.07g, 15mmol) in CCl4 (10mL) and CH2Cl2 (40mL), was added hydrazine hydrate 80% (2.0g, 50mmol) at rt. After 15min at room temperature, a solution of (-)-menthyl chloroformate 2 (2.19g, 10mmol) in CH2Cl2 (10mL), was added dropwise. Next, the mixture was stirred at rt for 3h. The crude mixture was washed with H2O (2×20mL) and extracted with CH2Cl2 (10mL), and the solvent was dried over MgSO4 and evaporated under vacuum. The crude product was purified by crystallization from hexanes to give (-)-menthyl carbazate (1.81g, 85% over 2 steps) as a white solid. White solid (1.81g, 85%); 1H NMR (400MHz, CDCl3) δ 5.93 (s, 1H), 4.59 (td, J=10.9, 4.4Hz, 1H), 3.74 (d, J=3.7Hz, 2H), 2.04 (dd, J=7.3, 4.6Hz, 1H), 1.90 (dtd, J=13.9, 7.0, 2.6Hz, 1H), 1.67 (ddd, J=9.2, 6.4, 3.2Hz, 2H), 1.49 (ttd, J=12.8, 6.5, 3.3Hz, 1H), 1.33 (t, J=11.4Hz, 1H), 1.24-0.71 (m, 13H).
References:
Li, Bing;Zhang, Shengyong;Chen, Weiping [Tetrahedron Asymmetry,2014,vol. 25,# 13-14,p. 1002 - 1007]

2216-51-5
704 suppliers
$5.00/100mg
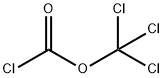
503-38-8
123 suppliers
$15.00/5g

14602-86-9
145 suppliers
$14.00/1g