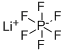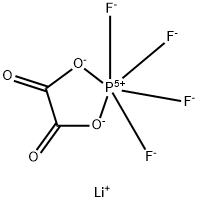
LiOTFP synthesis
- Product Name:LiOTFP
- CAS Number:521065-36-1
- Molecular formula:C2F4LiO4P
- Molecular Weight:201.93
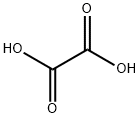
144-62-7
796 suppliers
$5.00/5g

521065-36-1
22 suppliers
inquiry
Yield: 99%
Reaction Conditions:
with lithium hexafluorophosphate;tetrachlorosilane at -50 - 25; for 2 h;Glovebox;Inert atmosphere;Reagent/catalyst;Solvent;Temperature;Concentration;
Steps:
1 Example 1
Example 1
[0032] In a glove box with a dew point of -50°C, 460 g of diethyl carbonate (DEC) dehydrated to a moisture of 10 massppm was introduced into a 1000-mL three-neck flask, and 76.0 g of lithium hexafluorophosphate (LiPF6) was addedthereto and dissolved therein while stirring. Subsequently, 45.0 g of oxalic acid dehydrated to a moisture of 150 massppm was added thereto. The three-neck flask was taken out from the glove box and was then immersed in a water bathset at 25°C, and the mixture was sufficiently stirred. Subsequently, 42.5 g of silicon tetrachloride was introduced into aflask with a cock, and a septum was attached to the inlet. A cannula was stuck into the septum, and the cock was openedto introduce a nitrogen gas into the flask for pneumatically transporting silicon tetrachloride into the mixture solution oflithium hexafluorophosphate, oxalic acid, and diethyl carbonate through the cannula over an hour by dropwise addition.Gases of silicon tetrafluoride and hydrogen chloride were generated coincidently with the start of the dropwise addition.The generated gases were sent to a can loaded with soda lime and were absorbed by the soda lime. The dissolution ofthe undissolved oxalic acid was caused, and the reaction further proceeded. After the completion of the addition, thestirring was continued for an hour to complete the reaction. In this example, the addition ratio of lithium hexafluorophosphate(shown in the column of "addition ratio of hexafluorophosphate" in Table 1) was controlled such that the amountof lithium hexafluorophosphate was 2.00 moles per 1 mole of silicon tetrachloride, and the addition ratio of oxalic acid(shown in the column of "addition ratio of oxalic acid" in Table 1) was controlled such that the amount of oxalic acid was2.00 moles per 1 mole of silicon tetrachloride. Diethyl carbonate was distilled away from the resulting reaction solutionat 50°C under a reduced pressure of 133 Pa. Subsequently, the three-neck flask was placed in a glove box, and thediethyl carbonate solution was filtered through a membrane filter. Several drops of the filtrate were introduced into anNMR tube, an internal standard was added thereto, and Acetonitrile-d3 was further added thereto and dissolved therein,followed by NMR measurement. The content percentage of each compound contained in the filtrate was calculated fromthe integral ratio of the NMR data. The filtrate contained 30 mass% of lithium tetrafluoro(oxalate)phosphate and 0.2mass% of lithium hexafluorophosphate. The yield of the lithium tetrafluoro(oxalate)phosphate was 99% based on thecharged quantity of lithium hexafluorophosphate.
References:
Central Glass Company, Limited;KUBO, Makoto;MORINAKA, Takayoshi;NAKAHARA, Keita;MURAMOTO, Satoshi EP2857408, 2015, A1 Location in patent:Paragraph 0032
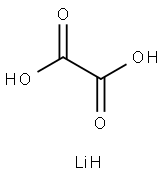
553-91-3
187 suppliers
$14.00/1g

521065-36-1
22 suppliers
inquiry
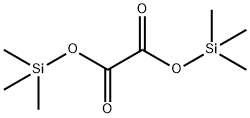
18294-04-7
8 suppliers
inquiry

521065-36-1
22 suppliers
inquiry