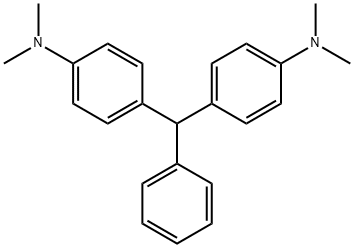
LEUCOMALACHITE GREEN synthesis
- Product Name:LEUCOMALACHITE GREEN
- CAS Number:129-73-7
- Molecular formula:C23H26N2
- Molecular Weight:330.47
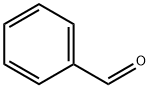
100-52-7
970 suppliers
$17.30/2g
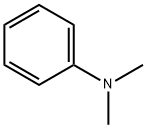
121-69-7
559 suppliers
$10.00/25mL

129-73-7
169 suppliers
$15.50/2g
Yield:129-73-7 85%
Reaction Conditions:
with N,N-dimethylformamidium hydrogen sulfate in neat (no solvent) at 120; for 4 h;Catalytic behavior;Reagent/catalyst;Temperature;
Steps:
General procedure for the synthesis of 3a-3i and 6j-6l
General procedure: To a mixture of aromatic aldehyde (2.0 mmol) and N,N-dimethylaniline or indole (5.0 mmol), ionic liquid AIL (0.2 mmol) was added, and the mixture was heated on an oil bath at 120 C with good stirring. After the completion of the reaction, monitored by thin-layer chromatography (TLC), the mixture was diluted with 5 cm3 ethyl acetate and 1 cm3 water. The organic layer was separated, dried over magnesium sulfate, and concentrated under reduced pressure. The residue was recrystallized with ethanol and afforded the corresponding TRAMs 3a-3h and 6j-6l in good isolated yields. Or the residue was purified by silica gel column chromatography (200-400 mesh silica gel, 1:8 petroleum ether/ethyl acetate) to afford 3i. The aqueous layer containing the AIL was subjected to rotary evaporation at 60 C under reduced pressure to afford the recovered AIL. Also, the catalyst could be reused easily six times without apparent loss of activity in terms of yield (85-83%; Fig. 3).
References:
Kang, Li Q.;Gao, Han;Cai, Yue Q. [Monatshefte fur Chemie,2018,vol. 149,# 1,p. 57 - 62]
![Benzoic acid, 2-[bis[4-(dimethylamino)phenyl]methyl]-](/CAS/20210305/GIF/62632-80-8.gif)
62632-80-8
0 suppliers
inquiry

129-73-7
169 suppliers
$15.50/2g

100-52-7
970 suppliers
$17.30/2g

121-69-7
559 suppliers
$10.00/25mL

129-73-7
169 suppliers
$15.50/2g
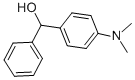
7494-77-1
27 suppliers
$155.00/50mg