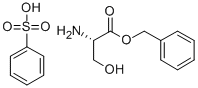
L-SERINE BENZYL ESTER BENZENESULFONATE ( synthesis
- Product Name:L-SERINE BENZYL ESTER BENZENESULFONATE (
- CAS Number:3695-68-9
- Molecular formula:C10H13NO3.C6H6O3S
- Molecular Weight:353.393
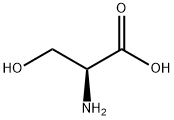
56-45-1
765 suppliers
$5.00/25g
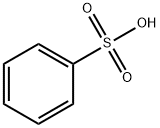
98-11-3
387 suppliers
$19.89/100g
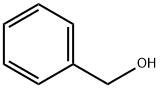
100-51-6
1402 suppliers
$5.00/100g

3695-68-9
12 suppliers
$69.29/5G
Yield:3695-68-9 85%
Reaction Conditions:
in benzene; for 5 h;Inert atmosphere;Reflux;
Steps:
4.2. Benzyl 2-(S)-[(tert-butoxycarbonyl)amino]-3-hydroxypropanoate 7
A mixture of l-serine (5.25 g, 50 mmol, 1.0 equiv), benzenesulfonic acid·hydrate (10.84 g, 62 mmol, 1.2 equiv), and benzyl alcohol (25 mL) was stirred in benzene (50 mL) with a Dean-Stark apparatus for 5 h. After removal of the remaining solvent by distillation, Et2O (100 mL) was added to the reaction mixture with vigorous shaking for 2 h until the mixture gave a solid. Storage of the resulting mixture at 4 °C overnight yielded a solid product. After filtration, the residue was washed with cold Et2O, and then dried. Recrystallization in hot EtOH gave l-serine benzyl ester benzenesulfonate (14.94 g, 42 mmol, >85%); 1H NMR (300 MHz, CDCl3) δ 8.12 (3H, m, inlMMLBox), 7.83 (2H, d, J = 7.2 Hz, o-inlMMLBox), 7.18-7.39 (8H, m, Bn, m,p-inlMMLBox), 5.08 (1H, d, J = 12.3 Hz, Bn), 5.02 (1H, d, J = 12.3 Hz, Bn), 4.30 (1H, s, OH), 4.16 (1H, s, CH), 4.02 (1H, dd, J = 12.3, 4.5 Hz, CH2), 3.90 (1H, dd, J = 12.3, 4.5 Hz, CH2).
References:
Koseki, Yohei;Yamada, Haruka;Usuki, Toyonobu [Tetrahedron Asymmetry,2011,vol. 22,# 5,p. 580 - 586] Location in patent:experimental part