L-KAWAIN synthesis
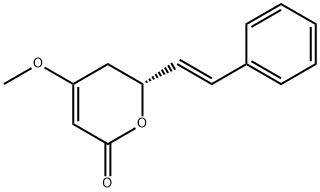
- Product Name:L-KAWAIN
- CAS Number:500-64-1
- Molecular formula:C14H14O3
- Molecular Weight:230.26
14371-10-9
319 suppliers
$6.00/25g
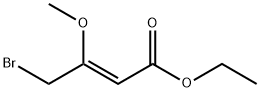
87830-18-0
0 suppliers
inquiry

500-64-1
106 suppliers
$28.00/10mg
Yield:-
Reaction Conditions:
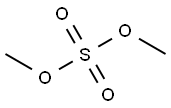
in benzene;
Steps:
1 EXAMPLE 1
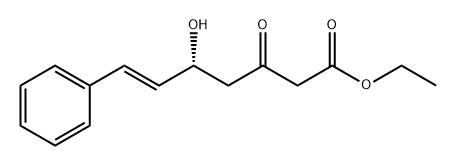
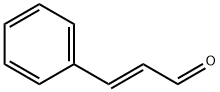
A 0.5M solution of ethyl γ-bromo-β-methoxycrotonate (1 eq.) in benzene is poured into a flask containing zinc filings (1.2 eq.). Cinnamic aldehyde (1.2 eq.) is added. Upon gentle warming to initiate the reaction, the mixture is refluxed for ca. 1 hr. The mixture is cooled, poured into cooled saturated aqueous ammonium chloride, and the aqueous phase extracted three times with ethyl ether. The combined extracts are dried over sodium sulfate, filtered and concentrated in vacuo. The resulting residue is recrystallized (MeOH) to give to give the desired product whose identity is confirmed by various means including proton nuclear magnetic resonance spectrometry and mass spectrometry.
References:
US2002/192241,2002,A1 US2003/181515,2003,A1 US2002/187169,2002,A1
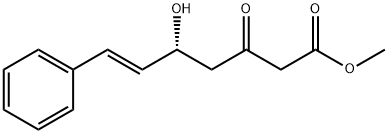
356793-34-5
0 suppliers
inquiry

500-64-1
106 suppliers
$28.00/10mg