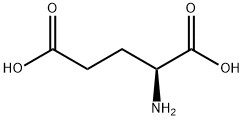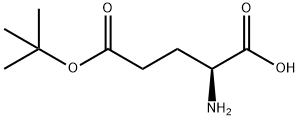
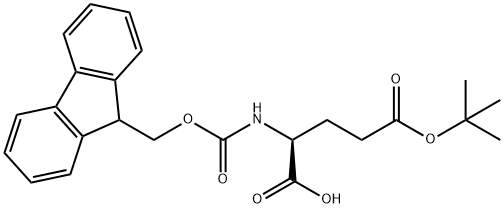
L-Glutamic acid 5-tert-butyl ester synthesis
- Product Name:L-Glutamic acid 5-tert-butyl ester
- CAS Number:2419-56-9
- Molecular formula:C9H17NO4
- Molecular Weight:203.24
71989-18-9
442 suppliers
$7.00/5g

2419-56-9
261 suppliers
$5.00/1g
Yield:-
Reaction Conditions:
with piperidine in N,N-dimethyl-formamide for 0.5 h;
Steps:
Fmoc-Glu(O-t-Bu)-OH (compound 19, 67mg, 0.156 mmol) was treated with piperidine (20%) in DMF (1 mL) for 30 min. The solvent was removed in vacuo to afford the crude primary amine. A solution of 4-carboxy-Pennsylvania Green succinimidyl ester3 (25 mg, 0.052 mmol) in DMF (1 mL) was added to the crude primary amine in DMF (1 mL) followed by DIEA (0.25 mL). The reaction was allowed to stir at 22 0C for 16 h, dried in vacuo, and purified by flash column chromatography (MeOH, CH2C12, AcOH, 5:94.9:0.1) to afford compound 20 (27 mg, 90%) as an orange residue, mp 134- 138 0C; IH NMR (400 MHz, CD3OD) δ 8.00 (s, IH), 7.94 (dd, J = 7.9, 1.2 Hz, IH), 7.40 (d, J = 7.9 Hz, IH), 6.98 - 6.89 (m, 2H), 6.81 - 6.77 (m, 2H), 4.70 (dd, J = 9.6, 4.9 Hz, IH), 2.47 (t, J = 7.0 Hz, 2H), 2.39 - 2.27 (m, IH), 2.14 - 2.08 (m, IH), 2.12 (s, 3H), 1.46 (s, 9H); 13C NMR (101 MHz, CD3OD) δ 174.95, 173.97, 169.66, 156.19, 138.02, 137.15, 136.55, 131.01, 130.47, 126.62, 112.98, 112.76, 106.32, 81.94, 53.70, 32.95, 28.35 (t), 27.66, 19.60 ; IR (film) v max 2929, 1678, 1607, 1536, 1371, 1302, 1190, 1139, 953, 842, 801, 750, 724 cm-1; HRMS (ESI+) m/z 568.1779 (M+H+, C30H27F2NO8, requires 568.1705).
References:
UNIVERSITY OF KANSAS;THE PENN STATE RESEARCH FOUNDATION;PETERSON, Blake R. WO2011/19942, 2011, A2 Location in patent:Page/Page column 31-32
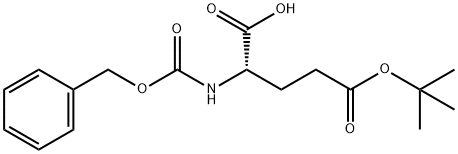
3886-08-6
219 suppliers
$5.00/1g

2419-56-9
261 suppliers
$5.00/1g
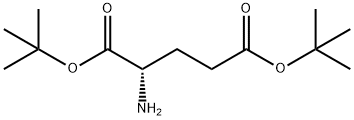
16874-06-9
33 suppliers
inquiry

2419-56-9
261 suppliers
$5.00/1g