
Indium chloride synthesis
- Product Name:Indium chloride
- CAS Number:10025-82-8
- Molecular formula:Cl3In
- Molecular Weight:221.18
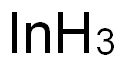
7440-74-6
267 suppliers
$28.00/1g
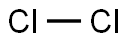
7782-50-5
0 suppliers
$405.00/454g

10025-82-8
283 suppliers
$7.00/1g
Yield:-
Reaction Conditions:
in dry Cl2-flow;
References:
Klemm, W. [Zeitschrift fur anorganische Chemie,1926,vol. 152,p. 253 - 254] Mathers, F. C. [Chemische Berichte,1907,vol. 40,p. 1231 - 1232] Mathers, F. C. [Journal of the American Chemical Society,1907,vol. 29,p. 493] Meyer, V.;Meyer, C. [Chemische Berichte,1879,vol. 12,p. 612] Winkler, C. [Journal fur praktische Chemie (Leipzig 1954),1867,vol. 102,p. 296] [Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: In: MVol., 3.6.3.1, page 79 - 79]
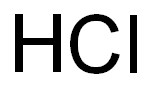
7647-01-0
0 suppliers
$10.00/10g
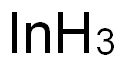
7440-74-6
267 suppliers
$28.00/1g

10025-82-8
283 suppliers
$7.00/1g
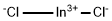
29790-97-4
0 suppliers
inquiry

10025-82-8
283 suppliers
$7.00/1g