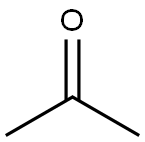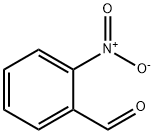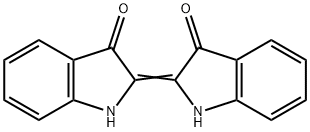
Indigo synthesis
- Product Name:Indigo
- CAS Number:482-89-3
- Molecular formula:C16H10N2O2
- Molecular Weight:262.26
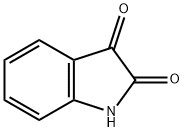
91-56-5
467 suppliers
$5.00/25g

482-89-3
402 suppliers
$13.00/100g
Yield:-
Steps:
Multi-step reaction with 2 steps
1: PCl5 / benzene / 4 h / Heating
2: 70 percent / thiophenol / benzene / 4 h / Heating; further thiophenols used: 1) p-CH3C6H4SH, 2) p-ClC6H4SH, 3) m-CH3C6H4SH, 4) o-CH3C6H4SH
References:
Katritzky, Alan R.;Fan, Wei-Qiang;Koziol, Anna E.;Palenik, Gus J. [Journal of Heterocyclic Chemistry,1989,vol. 26,p. 821 - 828]
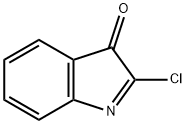
612-54-4
4 suppliers
inquiry

482-89-3
402 suppliers
$13.00/100g
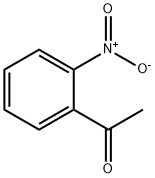
577-59-3
277 suppliers
$6.00/5g

482-89-3
402 suppliers
$13.00/100g
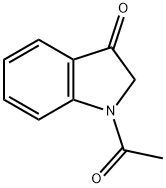
16800-68-3
119 suppliers
$21.00/250mg
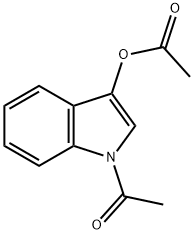
16800-67-2
146 suppliers
$15.00/250mg
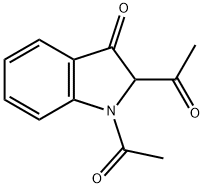
110912-08-8
0 suppliers
inquiry

482-89-3
402 suppliers
$13.00/100g