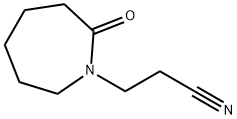
Hexahydro-2-oxo-1H-azepine-1-propanenitrile synthesis
- Product Name:Hexahydro-2-oxo-1H-azepine-1-propanenitrile
- CAS Number:7336-15-4
- Molecular formula:C9H14N2O
- Molecular Weight:166.22
Yield:7336-15-4 96.9 %
Reaction Conditions:
with sodium hydroxide in 5,5-dimethyl-1,3-cyclohexadiene at 45 - 80;Inert atmosphere;chemoselective reaction;Solvent;
Steps:
1; 5 Example 1: Reaction between ε-caprolactam and acrylonitrile in xylene
123.3 g of ε-caprolactam and 62.5 g of xylene were placed in a 1 l flask equipped with nitrogen inlet, stirrer, reflux condenser, thermocouple and dropping funnel. Under a light flow of nitrogen, the suspension was heated while stirring at 45-50°C using an oil bath; once completely solubilised, 0.1841 g of NaOH was added and the temperature was brought to 70°C. Once the sodium hydroxide was solubilised, acrylonitrile (67.4 g) was dripped, taking care to maintain the temperature between 70 and 80°C; the reaction was exothermic (estimated addition time about 1 h). At the end of the addition of the acrylonitrile, the temperature was kept at 70°C and the reaction was allowed to continue for 2.25 h. A progressive darkening of the solution was observed as the addition reaction progressed. GC-MS analysis showed a caprolactam conversion of 98.6%, a selectivity of 98.3% and thus a product yield of 96.9%. The basic raw solution was subjected to hydrogenation as described below in example 2.
References:
WO2022/189911,2022,A1 Location in patent:Page/Page column 23; 25-27

123-91-1
708 suppliers
$17.00/25g
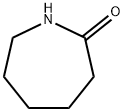
105-60-2
554 suppliers
$5.00/25g
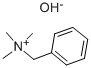
100-85-6
342 suppliers
$9.00/25g

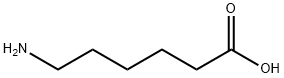
107-13-1
424 suppliers
$17.00/25ML

7336-15-4
33 suppliers
inquiry