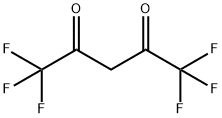
HEXAFLUOROACETYLACETONE synthesis
- Product Name:HEXAFLUOROACETYLACETONE
- CAS Number:1522-22-1
- Molecular formula:C5H2F6O2
- Molecular Weight:208.06
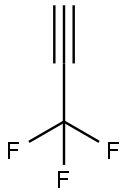
661-54-1
55 suppliers
$195.00/5 g
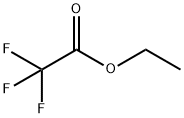
383-63-1
499 suppliers
$10.00/25 g

1522-22-1
282 suppliers
$6.00/1g
Yield:1522-22-1 2.7 g
Reaction Conditions:
Stage #1:3,3,3-trifluoroprop-1-yne with n-butyllithium in tetrahydrofuran;hexane at -30; for 0.25 h;Inert atmosphere;
Stage #2:ethyl trifluoroacetate, in tetrahydrofuran;hexane at -30; for 2 h;Inert atmosphere;
Stage #3: with sulfuric acid in tetrahydrofuran at 10; for 3.58 h;
Steps:
1
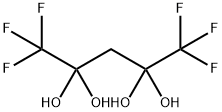
A solution I was prepared by adding 5.1 g (36 mmol, 1.2 eq) of ethyl trifluoroacetate of the following formula to 50 mL of tetrahydrofuran and cooling the resulting mixture to -30° C. while stirring. On the other hand, a solution II was prepared by adding 4.0 g (43 mmol, 1.4 eq) of 3,3,3-trifluoropropyne of the following formula to 50 mL of tetrahydrofuran at 0° C., further adding thereto 19 mL (30 mmol, 1.0 eq) of n-butyl lithium/n-hexane solution (1.6 M) at -30° C., and then, stirring the resulting mixture for 15 minutes at the same temperature as above. The solution I was mixed with the solution II. The mixed solution was stirred at -30° C. for 2 hours (as a reaction completed solution). The operation until this point was performed under a nitrogen atmosphere. The reaction completed solution was added to 78 g of aqueous sulfuric acid solution (prepared from 15 g (150 mmol, 5.0 eq) of concentrated sulfuric acid and 63 g of ice water) at 0° C. The thus-mixed solution was stirred at 50° C. for 10 hours (in a two-phase system) and separated into two phases. The aqueous layer was extracted with tert-butyl methyl ether. Then, the extract was combined with the organic phase. The recovered organic phase was subjected to F-NMR quantification by internal standard method (internal standard material: α,α,α-trifluorotoluene). It was confirmed that 25 mmol of 1,1,1,5,5,5-hexafluoroacetylacetone dihydrate of the following formula and 0.60 mmol of 1,1,1,5,5,5-hexafluoroacetylacetone monohydrate of the following formula were contained in the recovered organic phase. The total yield of the target product was 87%. Further, the recovered organic phase was washed with 20 mL of water and concentrated under a vacuum (40° C./60 mmHg). To the concentration residue, 18 mL of toluene was added. The resulting mixture was stirred for 3 hours and 10 minutes under ice cooling, thereby forming a crystalline precipitate. The crystalline precipitate was filtered out and dried under a vacuum. By this, 4.0 g of 1,1,1,5,5,5-hexafluoroacetone hydrate (dihydrate:monohydrate=97:3) was yielded. The total yield was 53%. To 3.4 g (14 mmol, 1 eq) of the above-obtained 1,1,1,5,5,5-hexafluoroacetone hydrate, 6.8 g (69 mmol, 4.9 eq) of concentrated sulfuric acid was added at 10° C. The resulting mixture was stirred for 3 hours and 35 minutes at room temperature (in a two-phase system). The thus-dehydrated solution was separated into two phases. By this, 2.7 g of 1,1,1,5,5,5-hexafluoroacetylacetone of the following formula was yielded. The recovery rate was 93%.
References:
JSI SiliconCo., Ltd.;Jung, Ir Nam;Kim, Uhn Sung;Kim, Sung Min;Kim, Young Min KR2015/130433, 2015, A Location in patent:Paragraph 0150-0181
428-75-1
8 suppliers
inquiry

1522-22-1
282 suppliers
$6.00/1g
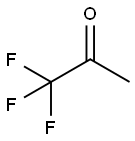
421-50-1
218 suppliers
$15.00/5 g

383-63-1
499 suppliers
$10.00/25 g

1522-22-1
282 suppliers
$6.00/1g
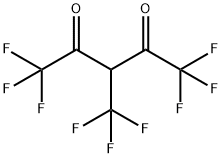
69962-11-4
0 suppliers
inquiry
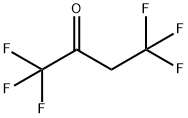
400-49-7
19 suppliers
$65.00/100mg

1522-22-1
282 suppliers
$6.00/1g
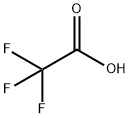
76-05-1
714 suppliers
$9.00/1g