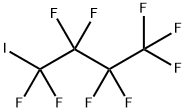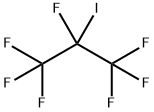
Heptafluoroisopropyl iodide synthesis
- Product Name:Heptafluoroisopropyl iodide
- CAS Number:677-69-0
- Molecular formula:C3F7I
- Molecular Weight:295.93
Yield:-
Reaction Conditions:
aluminum chlorofluoride
Steps:
4 Reaction of perfluorobutyl iodide with hexafluoropropene
Example 4 Reaction of perfluorobutyl iodide with hexafluoropropene A 240 mL Hastelloy shaker tube was flushed with nitrogen, then loaded with 3.5 g of aluminum chlorofluoride catalyst, cooled to -78° C., evacuated and loaded with 34.6 g (0.1 mole) of perfluorobutyl iodide and 15 g (0.1 mole) of hexafluoropropene. The reaction vessel was heated to 80° C. and kept on a shaker at 80° C. autogenous pressure for 24 hours. After 24 hours, the contents of the first shaker tube was transferred to a second shaker tube containing 3.5 g of aluminum chlorofluoride catalyst. Additional hexafluoropropene, 7.5 g 0.05 mole, was added and the tube was shaken at 80° C. for 48 hours. The crude product was according to gas chromatography and 19 F NMR, a mixture of perfluorobutene-2 and perfluoroisopropyl iodide. Upon distillation, 10 g of material with boiling point -3 to +2° C. was obtained that was according to 19 F NMR 97% pure perfluorobutene-2, mixture of cis and trans isomers in a 81:19 ratio. The residue (33 g) according to 19 F NMR contained 71% of perfluoroisopropyl iodide, 13% of perfluorobutene-2 and 16% of perfluorobutyl iodide starting material and a small amount of 2-iodoperfluorobutane as a contaminant. The calculated yield of perfluorobutene-2 was 84%, the calculated yield of perfluoroisopropyl iodide was 80%.
References:
E. I. du Pont de Nemours and Company US5929293, 1999, A
116-15-4
169 suppliers
$40.00/25 g

677-69-0
262 suppliers
$10.00/1g
3248-60-0
1 suppliers
inquiry

677-69-0
262 suppliers
$10.00/1g
3248-60-0
1 suppliers
inquiry

677-69-0
262 suppliers
$10.00/1g
116-14-3
49 suppliers
inquiry
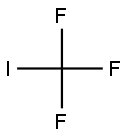
2314-97-8
184 suppliers
$40.00/25 g
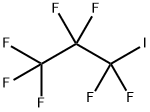
754-34-7
140 suppliers
$15.00/2 g

677-69-0
262 suppliers
$10.00/1g