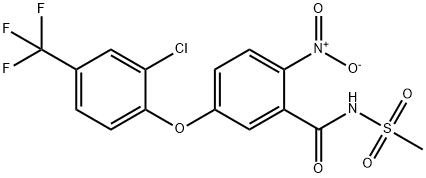
Fomesafen synthesis
- Product Name:Fomesafen
- CAS Number:72178-02-0
- Molecular formula:C15H10ClF3N2O6S
- Molecular Weight:438.76
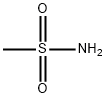
3144-09-0
375 suppliers
$6.00/5g
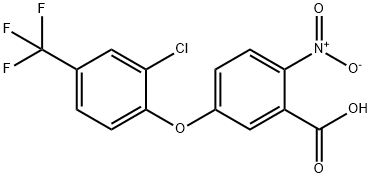
50594-66-6
200 suppliers
$78.00/1g

72178-02-0
226 suppliers
$44.79/1G
Yield:-
Steps:
1 EXAMPLE 1
EXAMPLE 1 This Example illustrates the preparation of compound no 1 of Table 1. 5(2-chloro-4-trifluoromethylphenoxy)-2-nitrobenzoic acid (1.58 g) was heated under reflux in an excess of thionyl chloride (20 ml) for 90 minutes. The excess of thionyl chloride was removed in a vacuum and the remaining oil taken up in dry pyridine (20 ml). Methanesulphonamide (0.45 g) was added and the mixture stirred at room temperature overnight. The pyridine was removed in a vacuum and the remaining oil mixed with 2-molar hydrochloric acid and extracted with ether (2*100 ml). The ether extracts were washed with water (100 ml), dried, and evaporated in a vacuum. The residual solid was recrystallized from isopropanol to give 5-(2-chloro-4-trifluoromethylphenoxy)-2-nitro-N-methanesulphonyl benzamide (Compound no 1) with a melting point of 201° C. In a similar way, but using the appropriate carboxy-substituted diphenyl ether and the appropriate sulphonamide in place of methanesulphonamide, the compounds listed in Table 1 were prepared, except for compounds 6, 8 and 10, whose preparation is described in Example 3.
References:
Imperial Chemical Industries PLC US4388472, 1983, A