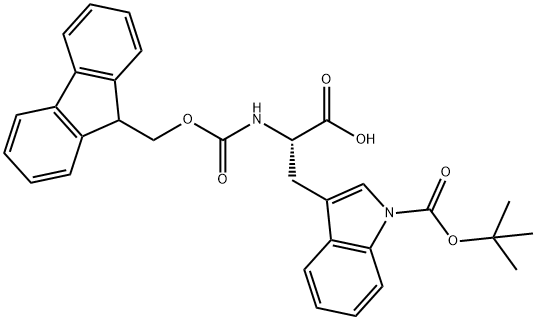
Fmoc-Trp(Boc)-OH synthesis
- Product Name:Fmoc-Trp(Boc)-OH
- CAS Number:143824-78-6
- Molecular formula:C31H30N2O6
- Molecular Weight:526.58
Yield:-
Reaction Conditions:
with calcium diiodide;sodium hydroxide in lithium hydroxide monohydrate;propan-2-one at 20; for 24 h;Green chemistry;
Steps:
2.3. Standard Conditions for Ester Hydrolysis
General procedure: In a 24-well plate (model 931565-G-1X from Thomson Instrument, Oceanside, CA,USA), a magnetic stir bar and 100 mol of amino ester (1 eq.) were added, followed by600 L of a 5 M solution of CaI2 (30 eq.), resulting in an insoluble mixture. Seventy-fivemicroliters of a 2 M NaOH solution (1.5 eq.) were then quickly added, forming a whiteprecipitate; then, 1.6 mL of organic solvent was subsequently added (1.6 mL/100 molamino ester), resulting in a 2.3:1 organic solvent for aqueous mixture. The 24-well plate wascovered using adhesive aluminum foil to avoid evaporation and left to stir for the desiredamount of time
References:
Binette, Renaud;Boudreault, Pierre-Luc;Desgagné, Michael;Theaud, Camille [Molecules,2022,vol. 27,# 9,art. no. 2788]
73-22-3
990 suppliers
$5.00/250mg

143824-78-6
421 suppliers
$10.00/1g
4299-70-1
41 suppliers
$337.00/250mg

143824-78-6
421 suppliers
$10.00/1g
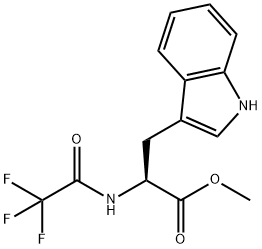
1604-49-5
13 suppliers
inquiry

143824-78-6
421 suppliers
$10.00/1g
![L-Tryptophan, 1-[(1,1-dimethylethoxy)carbonyl]-N-[(phenylmethoxy)carbonyl]-, methyl ester](/CAS/20211123/GIF/643751-42-2.gif)
643751-42-2
0 suppliers
inquiry

143824-78-6
421 suppliers
$10.00/1g
![tert-butyl 3-[(2S)-2-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}-3-methoxy-3-oxopropyl]indole-1-carboxylate](/CAS/20210305/GIF/405555-31-9.gif)