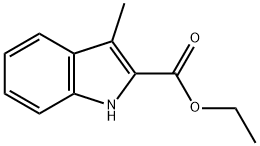
ETHYL3-METHYL-2-INDOLECARBOXYLATE synthesis
- Product Name:ETHYL3-METHYL-2-INDOLECARBOXYLATE
- CAS Number:26304-51-8
- Molecular formula:C12H13NO2
- Molecular Weight:203.24
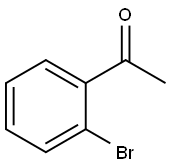
2142-69-0
325 suppliers
$6.00/1g
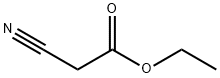
105-56-6
454 suppliers
$10.00/5ml

26304-51-8
60 suppliers
$18.00/100mg
Yield: 49%
Reaction Conditions:
with copper(l) iodide;caesium carbonate in dimethyl sulfoxide at 50;Inert atmosphere;
Steps:
3.1 Step 1:
Preparation of ethyl 3-methyl-1H-indole-2-carboxylate
2-bromoacetophenone (3g, 15.07mmol), CuI (287mg, 1.51mmol) and cesium carbonate (9.82g, 30.14mmol) were added to 40mL of DMSO, and ethyl cyanoacetate was slowly added at 50 °C under protect of nitrogen.
After the reaction was completed, 40 mL of water was added, and ethyl acetate (3*50 mL) was added for extraction.
The combined organic layer was washed with water (3*30 mL) and saturated NaCl solution (50 mL), dried over anhydrous Na2SO4 and filtered.
The solvent was dried under reduced pressure, and the residue was isolated and purified by flash column chromatography (petroleum ether / ethyl acetate = 4/1, v / v) to afford the subject product 1.5 g, yield 49%.MS (ESI, m/z): 204(M +H)+.
References:
Shanghai Institute of Materia Medica, Chinese Academy of Sciences;JIANG, Hualiang;LIU, Hong;GENG, Meiyu;ZHENG, Mingyue;AI, Jing;WANG, Yulan;WU, Xiaowei;LI, Shuangjie;PENG, Xia;LI, Chunpu;CHEN, Kaixian;WANG, Bao EP3470415, 2019, A1 Location in patent:Paragraph 0098; 0099
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

26304-51-8
60 suppliers
$18.00/100mg
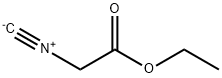
2999-46-4
219 suppliers
$13.43/1gm:

2142-69-0
325 suppliers
$6.00/1g

26304-51-8
60 suppliers
$18.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
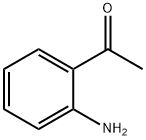
551-93-9
498 suppliers
$6.00/5g

26304-51-8
60 suppliers
$18.00/100mg

541-41-3
0 suppliers
$19.39/5g
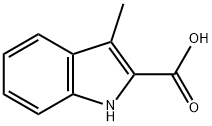
10590-73-5
70 suppliers
$46.00/100mg

26304-51-8
60 suppliers
$18.00/100mg
