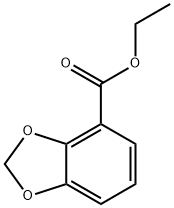
Ethyl benzo[d][1,3]dioxole-4-carboxylate synthesis
- Product Name:Ethyl benzo[d][1,3]dioxole-4-carboxylate
- CAS Number:23158-06-7
- Molecular formula:C10H10O4
- Molecular Weight:194.18

75-09-2
1218 suppliers
$10.00/25ML
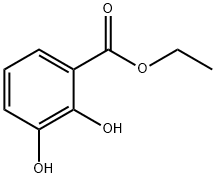
3943-73-5
69 suppliers
$16.00/100mg
![Ethyl benzo[d][1,3]dioxole-4-carboxylate](/CAS/20180601/GIF/23158-06-7.gif)
23158-06-7
6 suppliers
inquiry
Yield:23158-06-7 68%
Reaction Conditions:
with potassium fluoride in N,N-dimethyl-formamide at 110; for 14.5 h;
Steps:
68.2
To a solution of the compound of the previous step (22 g) in DMF (200 ml) were added potassium fluoride (36 g) and CH2Cl2 (9.0 ml), and the mixture was stirred at 110°C for 8 hr. CH2Cl2 (20 ml) was added to the solution, and the mixture was further stirred at 110°C for 6.5 hr. The reaction solution was poured into water, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was evaporated under reduced pressure. The residue was subjected to normal phase chromatography (elution solvent hexane-ethyl acetate=4:1) to give the title compound (16 g, 68%). NMR(300MHz, CDCl3)δ:1.39(3H, t, J=7.2Hz), 4.39(2H, q, J=7.2Hz), 6.10(2H, s), 6.86(1H, dd, J=8.1, 7.2Hz), 6.97(1H, dd, J=7.2, 1.2Hz), 7.41(1H, dd, J=8.1, 1.2Hz).
References:
EP2116538,2009,A1 Location in patent:Page/Page column 49
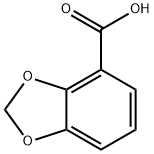
5768-39-8
108 suppliers
$54.00/100mg

64-17-5
728 suppliers
$10.00/50g
![Ethyl benzo[d][1,3]dioxole-4-carboxylate](/CAS/20180601/GIF/23158-06-7.gif)
23158-06-7
6 suppliers
inquiry
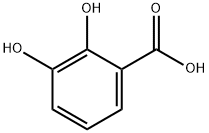
303-38-8
319 suppliers
$15.00/5g
![Ethyl benzo[d][1,3]dioxole-4-carboxylate](/CAS/20180601/GIF/23158-06-7.gif)
23158-06-7
6 suppliers
inquiry
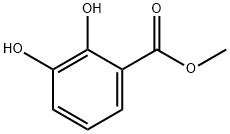
2411-83-8
103 suppliers
$13.00/1g
![Ethyl benzo[d][1,3]dioxole-4-carboxylate](/CAS/20180601/GIF/23158-06-7.gif)
23158-06-7
6 suppliers
inquiry
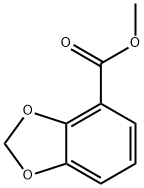
33842-16-9
89 suppliers
$45.00/100mg
![Ethyl benzo[d][1,3]dioxole-4-carboxylate](/CAS/20180601/GIF/23158-06-7.gif)
23158-06-7
6 suppliers
inquiry