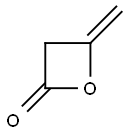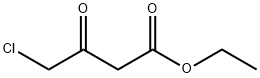
Ethyl 4-chloroacetoacetate synthesis
- Product Name:Ethyl 4-chloroacetoacetate
- CAS Number:638-07-3
- Molecular formula:C6H9ClO3
- Molecular Weight:164.59
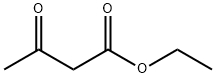
141-97-9
814 suppliers
$5.00/25g

638-07-3
502 suppliers
$5.00/5G
Yield:638-07-3 100%
Reaction Conditions:
Stage #1: ethyl acetoacetatewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at 0; for 1 h;Inert atmosphere;
Stage #2: with methyl chlorosulfate in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Steps:
Chlorination of lithium dienolates of 1,3-dicarbonyl compounds
General procedure: To a solution of diisopropylamine (340 μL, 2.4 eq.) in dry THF (2 mL) in a flame dried round bottom flask under argon at 0 °C was added n-butyllithium (1.4 mL, 1.6 M in hexanes, 2.2 eq.), and the reaction mixture was stirred at this temperature for 15 minutes. At the same temperature a solution of dicarbonyl compound (3n) (1 mmol) in THF (2 mL) was slowly added. After 1 hour of stirring at 0 °C the solution was cooled to -78 °C and methyl chlorosulfate (110 μL, 1.2 eq.) was then added. After stirring at -78 °C for 30 minutes, the reaction was quenched with saturated ammonium chloride aqueous solution (5 mL). The mixture was then extracted with dichloromethane (3 x 5 mL), the combined organic phases were dried with anhydrous magnesium sulfate and the solvent evaporated affording the desired 4-chloro dicarbonyl compound 5n.
References:
Silva, Saúl;Maycock, Christopher D. [Tetrahedron Letters,2018,vol. 59,# 13,p. 1233 - 1238] Location in patent:supporting information
141-78-6
652 suppliers
$22.37/250ml
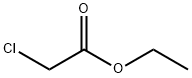
105-39-5
416 suppliers
$10.00/5g

638-07-3
502 suppliers
$5.00/5G

141-78-6
652 suppliers
$22.37/250ml
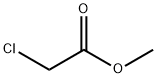
96-34-4
356 suppliers
$10.00/25g

638-07-3
502 suppliers
$5.00/5G