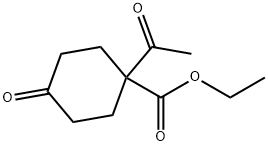
ethyl 1-acetyl-4-oxocyclohexane-1-carboxylate synthesis
- Product Name:ethyl 1-acetyl-4-oxocyclohexane-1-carboxylate
- CAS Number:72653-21-5
- Molecular formula:C11H16O4
- Molecular Weight:212.24
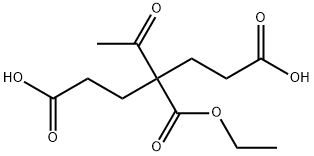
72653-14-6
46 suppliers
$96.00/1g

72653-21-5
29 suppliers
$175.00/100mg
Yield:72653-21-5 17%
Reaction Conditions:
with pyridine;acetic anhydride at 145; for 2 h;
Steps:
D28.3 Step 3: ethyl- l-acetyl-4-oxocyclohexanecarboxylate (D28-3)
To a stirred suspension of compound D28-2 (85 g, 310 mmol) in acetic anhydride (255 mL) was added pyridine (27 mL) and the mixture was stirred at 145 °C for 2 h. The solvent was removed under reduced pressure, the residue was suspended in water (200 mL) and extracted with EtOAc (3 x 100 mL). The combined organic layers were washed with water (100 mL), brine (100 mL), dried over Na2S04 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (14% EtOAc/hexane as eluent) to provide compound D28-3 (11 g, 17%) as brown gum. FontWeight="Bold" FontSize="10" H NMR (CDCI3, 400 MHz): δ 4.28 (q, J = 7.2 Hz, 2H), 2.44-2.42 (m, 6H), 2.23-2.20 (m, 5H), 1.31 (t, J = 7.2 Hz, 3H).
References:
WO2015/129926,2015,A1 Location in patent:Page/Page column 199
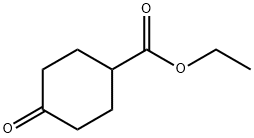
17159-79-4
345 suppliers
$5.00/1g

72653-21-5
29 suppliers
$175.00/100mg
![ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1489-97-0.gif)
1489-97-0
141 suppliers
$17.00/1g

72653-21-5
29 suppliers
$175.00/100mg
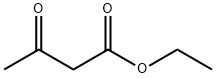
141-97-9
795 suppliers
$5.00/25g

72653-21-5
29 suppliers
$175.00/100mg
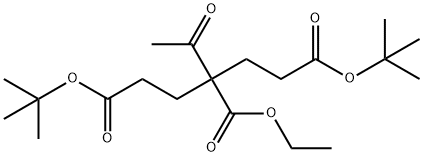
1135619-58-7
2 suppliers
inquiry

72653-21-5
29 suppliers
$175.00/100mg
