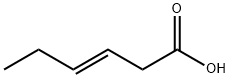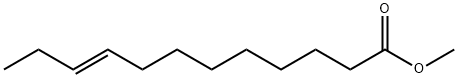
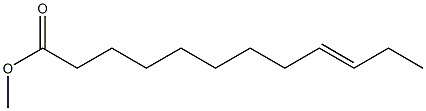
(E)-9-Dodecenoic acid methyl ester synthesis
- Product Name:(E)-9-Dodecenoic acid methyl ester
- CAS Number:55030-26-7
- Molecular formula:C13H24O2
- Molecular Weight:212.33

74-85-1
105 suppliers
$79.00/11l
39202-17-0
1 suppliers
inquiry
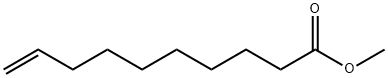
25601-41-6
48 suppliers
inquiry

55030-26-7
1 suppliers
inquiry
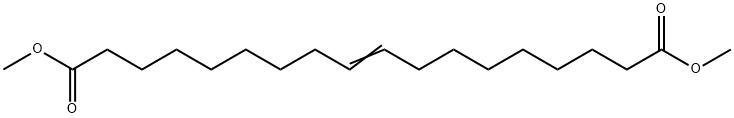
13481-97-5
1 suppliers
inquiry
Yield:55030-26-7 80 %Chromat.
Reaction Conditions:
with C46H52Br2Cl2N2O3SiW at 20; under 7500.75 Torr; for 2 h;Alkene (Olefin) Metathesis;
Steps:
1.1 Ethenolysis of a mixture of E- and Z-isomers of methyl 9-dodecenoate 1 and 2:
The stock solution of catalyst (prepared according to known methods; TBS = terf.-butyldimethylsilyl) was added to methyl 9-dodecenoate (0.45 ml_, EIZ = 80/20 mixture) and the reaction mixture was pressurized by 10 bar ethylene and stirred at rt for 2 h. Then the pressure was released and MeOH (4.5 ml) was added. An aliquot (200 μΙ_) was taken out and charged onto the top of a silica gel column (0.5 ml silica gel in a 20 ml hypodermic syringe) and the components were eluted with dichloromethane (total DCM used: 25 ml). The eluate was analyzed by gas chromatography. The E-isomer 1 was obtained in a yield of approx. 80 % having a stereoisomeric purity of approx. 96 %. 3 is the ethenolysis product of Z-isomer 2. Compound 4 is formed as a side-product.
References:
WO2018/220088,2018,A1 Location in patent:Paragraph 00119; 00121