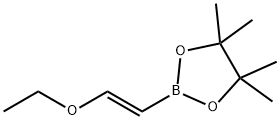
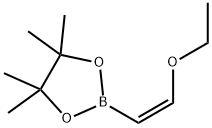
(E)-1-Ethoxyethene-2-ylboronic acid pinacol ester synthesis
- Product Name:(E)-1-Ethoxyethene-2-ylboronic acid pinacol ester
- CAS Number:1201905-61-4
- Molecular formula:C10H19BO3
- Molecular Weight:198.07
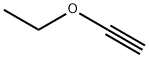
927-80-0
141 suppliers
$27.50/1g
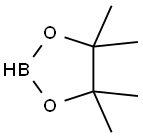
25015-63-8
220 suppliers
$22.39/5G

1201905-61-4
112 suppliers
$13.00/250mg
Yield:1201905-61-4 91%
Reaction Conditions:
with Schwartz's reagent in hexane;dichloromethane at 0 - 20; for 18 h;
Steps:
29 Preparation 29: (£)-2-(2-ethoxyvinyl)-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane
Preparation 29: (£)-2-(2-ethoxyvinyl)-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane A solution of ethoxyethyne (60% w/w in hexanes, 30 mL, 154 mmol) in DCM (230 mL) was cooled at 0°C and pinacol borane (27 mL, 186 mmol) was added followed by Cp2Zr(H)CI (1 .96 g, 7.60 mmol). The mixture was allowed to reach room temperature and stirred for 18 hours. The reaction was filtered through a pad of neutral alumina eluting with 1 0% EtOAc in cyclohexane to afford the title compound (27.66 g, 91 %). 1 H NMR (500 MHz, CDCI3) : δ 7.05 (d, J = 14.4 Hz, 1 H), 4.45 (d, J = 14.4 Hz, 1 H), 3.86 (q, J = 7.1 Hz, 2H), 1 .30 (t, J = 7.1 Hz, 3H), 1 .27 (s, 12H).
References:
CANCER RESEARCH TECHNOLOGY LIMITED;HOELDER, Swen;BLAGG, Julian;SOLANKI, Savade;WOODWARD, Hannah;NAUD, Sebastian;BAVETSIAS, Vassilios;SHELDRAKE, Peter;INNOCENTI, Paolo;CHEUNG, Jack;ATRASH, Butrus WO2014/37750, 2014, A1 Location in patent:Paragraph 0042-0045
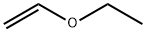
109-92-2
273 suppliers
$16.00/25mL

25015-63-8
220 suppliers
$22.39/5G

1201905-61-4
112 suppliers
$13.00/250mg
219489-07-3
41 suppliers
$58.00/250mg