DIMETHYL DIBROMOMALONATE synthesis
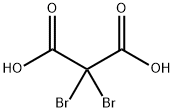
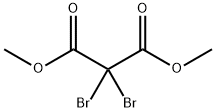
- Product Name:DIMETHYL DIBROMOMALONATE
- CAS Number:37167-59-2
- Molecular formula:C5H6Br2O4
- Molecular Weight:289.91
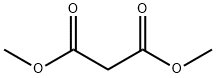
108-59-8
631 suppliers
$5.00/25g

37167-59-2
17 suppliers
$106.00/250mg
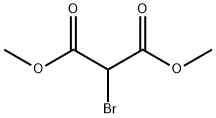
868-26-8
107 suppliers
$40.00/5g
Yield:-
Reaction Conditions:
with N-Bromosuccinimide;ethyl 2-methyl-1H-indole-3-carboxylate in n-heptane at 25; for 18 h;Darkness;Overall yield = 90 percentSpectr.;
Steps:
Representative procedure for the bromination of 1,3-dicarbonylcompound 9 using catalytic amount of indole
General procedure: To a suspension of indole 1d (3.0 mg, 0.015 mmol, 0.05 equiv)and methyl acetoacetate (9a) (32.4 l, 0.3 mmol, 1 equiv) inheptane (1.5 mL, 0.2 M) was added N-bromosuccinimide(56.1 mg, 0.315 mmol, 1.05 equiv) in the absence of light at 25 C. The resultant mixture was stirred at the same temperature for18 h. The reaction mixture was then filtered through a cotton plug.The filtrate was concentrated under reduced pressure and the yieldwas measured using NMR with internal standard.
References:
Wong, Jonathan;Ke, Zhihai;Yeung, Ying-Yeung [Tetrahedron Letters,2020,vol. 61,# 16,art. no. 151772]

108-59-8
631 suppliers
$5.00/25g

37167-59-2
17 suppliers
$106.00/250mg