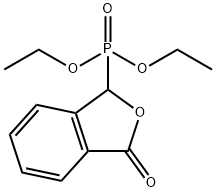
DIETHYL PHTHALIDE-3-PHOSPHONATE synthesis
- Product Name:DIETHYL PHTHALIDE-3-PHOSPHONATE
- CAS Number:17858-15-0
- Molecular formula:C12H15O5P
- Molecular Weight:270.22
Yield:-
Reaction Conditions:
with methanesulfonic acid;sodium 2,2-dimethylpropan-1-olate in 2-methyltetrahydrofuran at 2 - 20;
Steps:
4.a
Example 4: Alternative synthesis of Compound DE ED D(a) 2-Fluoro-5-[(E/Z)-(3-oxo-2-benzofuran- 1 (3H)-ylidene)methyl]benzonitrile (E) Sodium t-amylate (99.00 g, 0.854 mol) and 2-methyltetrahydrofuran (960 ml) were cooled to 2°C under a nitrogen atmosphere. Diethyl phosphite (110 ml, 0.855 mol) was added dropwise maintaining the temperature at <5°C. 2-Methyltetrahydrofuran (40 ml) was added as line wash. The reaction was stirred at 2°C for 1 hour 40 minutes. A solution of 2-carboxybenzaldehyde (H)(80 g, 0.533 mol) in 2-methyltetrahydrofuran (200 ml) was added, maintaining the temperature at <7°C throughout the addition. A line wash of 2-methyltetrahydrofuran (40 ml) was added. The reaction mixture was warmed to 200C and held at 200C for 20 minutes. Methanesulphonic acid (66 ml, 1.01 mol) was added over 1 hour and 10 minutes, followed by 2- methyltetrahydrofuran (40 ml). The reaction mixture was stirred at 20°C over night. Methanesulphonic acid (7 ml, 0.101 mol) was added, followed by 2-methyltetrahydrofuran (7 ml) and the reaction stirred at 2O0C for a further 4 hours. Water (400 ml) was added at room temperature and the resulting biphasic mixture stirred at room temperature for 20 minutes. The lower aqueous layer was removed and a solution of potassium bicarbonate (53.50 g, 0.534 mol) in water (400 ml) was added to the organic layer. The biphasic mixture was stirred at room temperature for 20 minutes and then the lower aqueous solution was removed. The organic fraction was retained (solution of diethyl (3-oxo1 ,3-dihydro-2-benzofuran-1-yl)phosphonate).
References:
WO2008/47082,2008,A2 Location in patent:Page/Page column 27
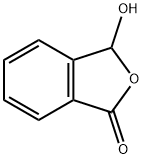
4741-67-7
0 suppliers
inquiry

17858-15-0
7 suppliers
inquiry
4376-18-5
182 suppliers
$9.00/5g

17858-15-0
7 suppliers
inquiry
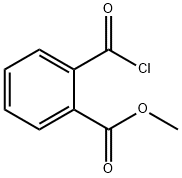
4397-55-1
23 suppliers
$49.47/500mgs:

17858-15-0
7 suppliers
inquiry
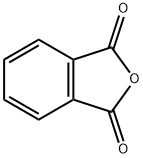
85-44-9
764 suppliers
$9.00/5g

17858-15-0
7 suppliers
inquiry