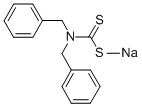
DIBENZYLDITHIOCARBAMIC ACID SODIUM SALT synthesis
- Product Name:DIBENZYLDITHIOCARBAMIC ACID SODIUM SALT
- CAS Number:55310-46-8
- Molecular formula:C15H14NNaS2
- Molecular Weight:295.4

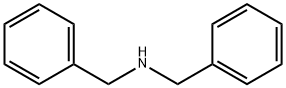
75-15-0
261 suppliers
$40.00/100 g
103-49-1
467 suppliers
$5.00/25g

55310-46-8
112 suppliers
$27.00/1g
Yield: 54%
Reaction Conditions:
Stage #1:dibenzylamine with sodium hydroxide in ethanol at 0; for 0.5 h;
Stage #2:carbon disulfide in ethanol for 3 h;
Steps:
2.2.1. Synthesis of dibenzyldithiocarbamato sodium salt, Na(dbzdtc)
Dibenzyldithiocarbamato sodium salt Na(dbzdtc) was prepared based on a literature[14] procedure with slight modification. Sodium hydroxide (0.05 mol) was dispersed in10 mL of ethanol and kept at 0 °C. Dibenzylamine (0.05 mol) was added to the ice-coldmixture at 0 °C and stirred for 30 min before adding 3mL of cold CS2 (0.5 mol) slowly.The reaction was left to stir for 3 h while maintaining the temperature. The productwas filtered and dried. Yield: 8.1189 g, (54%), m.p.t (°C) 123.3-124.8. Selected IR,(cm1): t(C-N) 1443, t(C2-N) 1346, t(C-S)Asym 1144, t(C-S)Sym 969. 1H NMR (D2O) δ/ppm 7.33-7.49 (m, C6H5), 5.42 (s, CH2), 13C NMR (D2O) δ=212.58 (CS2), 126.96-128.83 ppm, 136.79 ppm (C), 59.63 ppm (CH2). MS: m/z [M+] 272.1446. UV-Vispp (NCS) 261 nm, pp (SCS) 287 nm.
References:
Ajibade, Peter A.;Sikakane, Berlinda M.;Oluwalana, Abimbola E.;Paca, Athandwe M.;Singh, Moganavelli [Journal of Coordination Chemistry,2021,vol. 74,# 8,p. 1244 - 1254]