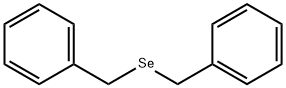
DIBENZYL SELENIDE synthesis
- Product Name:DIBENZYL SELENIDE
- CAS Number:1842-38-2
- Molecular formula:C14H14Se
- Molecular Weight:261.22
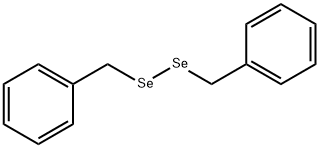
1482-82-2
135 suppliers
$17.89/1g
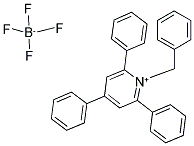
66310-10-9
25 suppliers
inquiry

1842-38-2
10 suppliers
$28.60/50mg
Yield:1842-38-2 98%
Reaction Conditions:
with eosin Y disodium salt;N-ethyl-N,N-diisopropylamine in acetonitrile at 20; for 20 h;Schlenk technique;Inert atmosphere;Irradiation;Green chemistry;
Steps:
General Procedure for Synthesis of.
General procedure: The corresponding pyridinium salt (0.4 mmol, 1.0 equiv), Eosin Y (0.48 mmol, 0.12 equiv) and Diselenides (0.48 mmol, 1.2 equiv) were weighed into a Schlenk tube containing a magnetic stirring bar. The tube was evacuated and backfilled with Argon three times. MeCN (2 mL, 0.2 M) and DIPEA (0.8 mmol, 2.0 equiv) was added and the reaction was stirred under irradiation with 24 W blue LEDs for 20 h at room temperature. the solvent was removed under reduced pressure. The crude product was purified by column chromatography over silica gel to afford pure products.
References:
Ji, Liangshuo;Qiao, Jiamin;Li, Ankun;Jiang, Zeyi;Lu, Kui;Zhao, Xia [Tetrahedron Letters,2021,vol. 72,art. no. 153071] Location in patent:supporting information
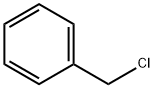
100-44-7
646 suppliers
$13.50/250G

1842-38-2
10 suppliers
$28.60/50mg

1482-82-2
135 suppliers
$17.89/1g
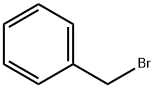
100-39-0
433 suppliers
$10.00/10g

1842-38-2
10 suppliers
$28.60/50mg

1482-82-2
135 suppliers
$17.89/1g

100-39-0
433 suppliers
$10.00/10g

1842-38-2
10 suppliers
$28.60/50mg

100-44-7
646 suppliers
$13.50/250G

1842-38-2
10 suppliers
$28.60/50mg