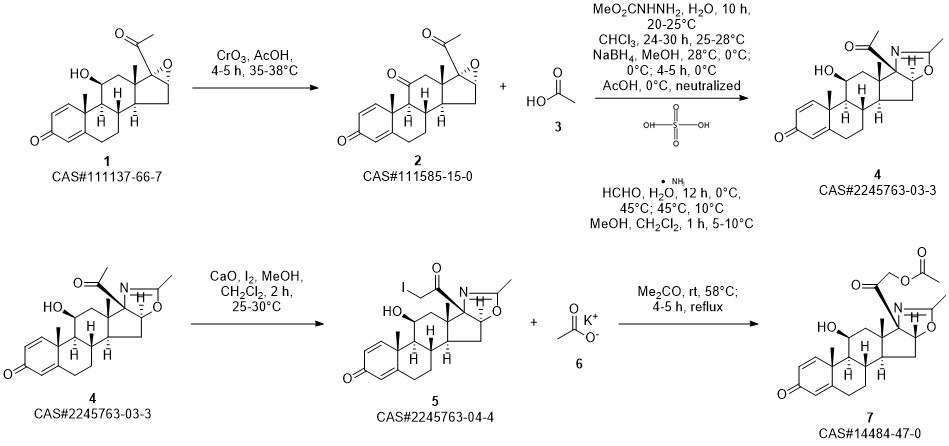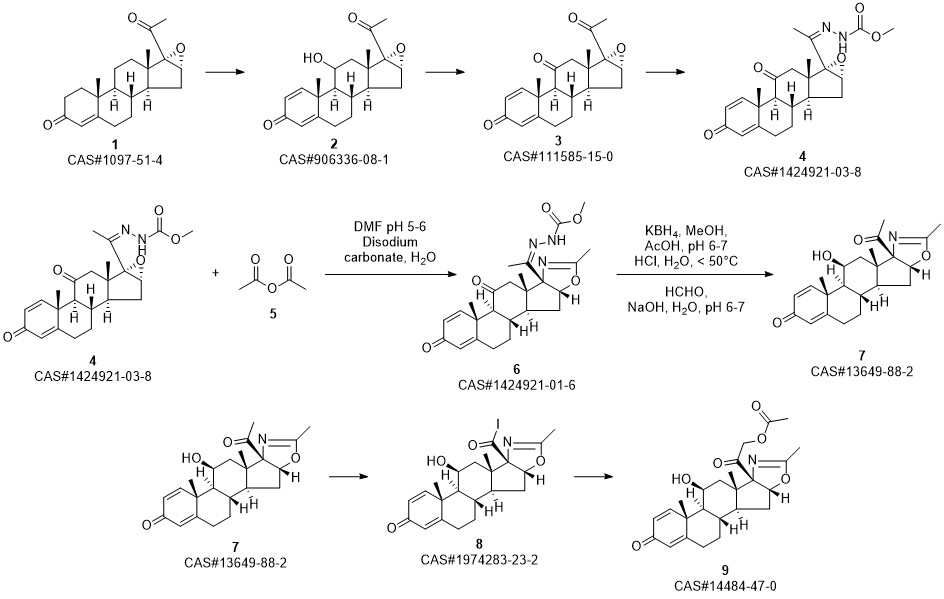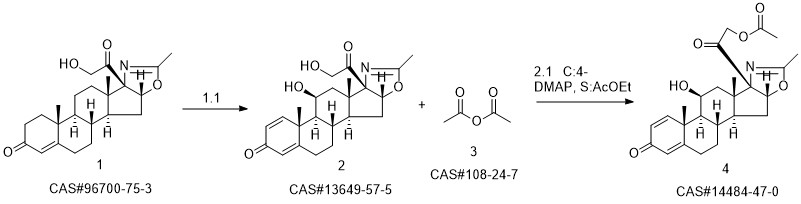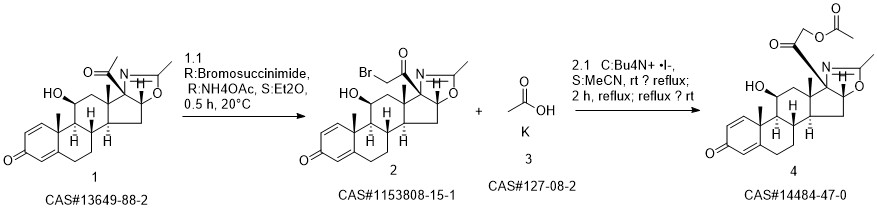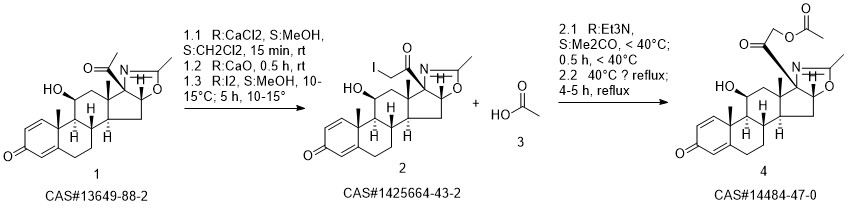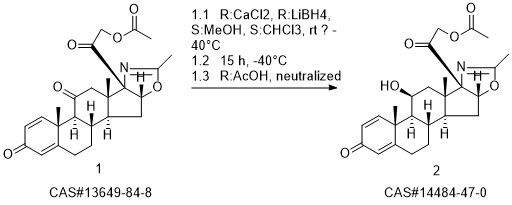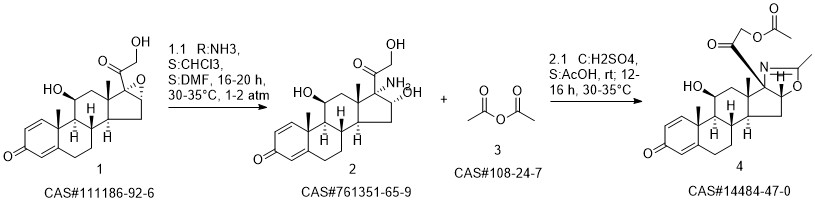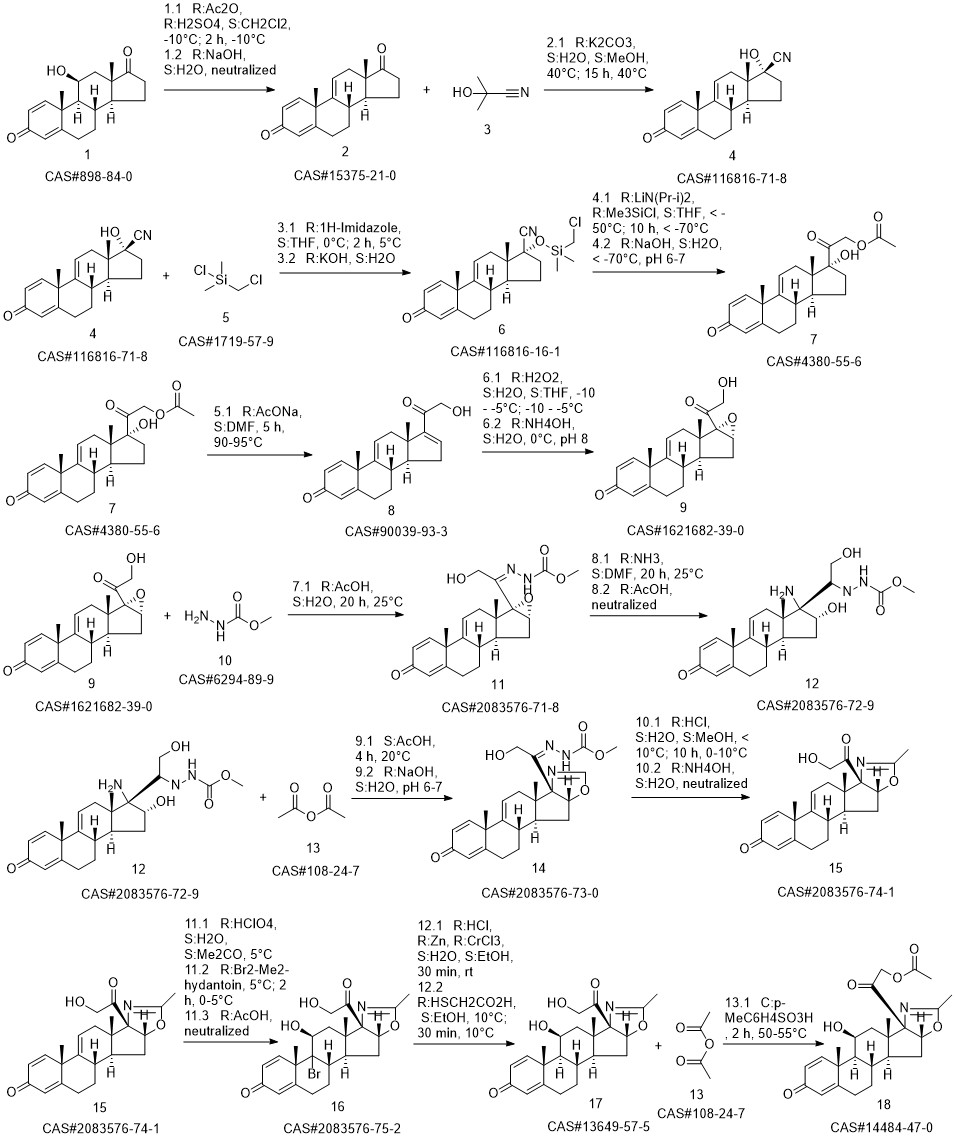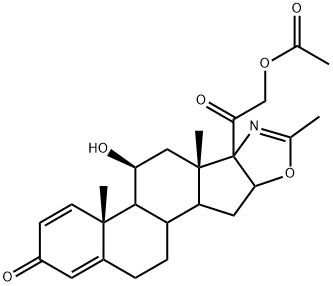
Deflazacort synthesis
- Product Name:Deflazacort
- CAS Number:14484-47-0
- Molecular formula:C25H31NO6
- Molecular Weight:441.52
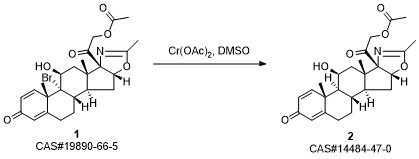
Reference: Nathansohn G, Winters G, Aresi V. Steroids possessing nitrogen atoms. IV. Further studies on the synthesis of [17alpha,16alpha-d]-oxazolino-corticoids. Steroids. 1969 Mar;13(3):383-97. PubMed PMID: 5789709.
![5'βH-5α-Pregnano[17,16-d]oxazol-20-one, 3β,11β-dihydroxy-2'-methyl-, 3-acetate (8CI)](/CAS/20240320/GIF/13649-87-1.gif)
13649-87-1
0 suppliers
inquiry

14484-47-0
469 suppliers
$28.00/25mg
Yield:-
Steps:
Multi-step reaction with 6 steps
1: MeSO2Cl, SO2, collidine / dimethylformamide
2: aq. KOH / methanol / Heating
3: (i) I2, CaO, AIBN, THF, MeOH, (ii) /BRN= 506007/, Et3N, acetone
4: aq. CrO3 / acetone
5: (i) Br2, aq. HBr, AcOH, dioxane, (ii) LiBr, Li2CO3, DMF
6: (i) AcNHBr, HClO4, THF, (ii) Cr(OAc)2, nBuSH, DMSO
References:
Nathansohn,G. et al. [Steroids,1969,vol. 13,p. 383 - 397]
![5'βH-5α-Pregnano[17,16-d]oxazol-20-one, 3β,11β-dihydroxy-2'-methyl- (8CI)](/CAS/20211123/GIF/13649-86-0.gif)
13649-86-0
0 suppliers
inquiry

14484-47-0
469 suppliers
$28.00/25mg
![5'βH-5α-Pregnano[17,16-d]oxazole-3,20-dione, 11β-hydroxy-2'-methyl- (8CI)](/CAS/20240320/GIF/14927-19-6.gif)
14927-19-6
0 suppliers
inquiry

14484-47-0
469 suppliers
$28.00/25mg
![5'βH-5α-Pregnano[17,16-d]oxazol-20-one, 3β,11β-dihydroxy-2'-methyl-, semicarbazone (8CI)](/CAS/20240320/GIF/13649-85-9.gif)
13649-85-9
0 suppliers
inquiry

14484-47-0
469 suppliers
$28.00/25mg
![5'βH-5α-Pregnano[17,16-d]oxazole-11,20-dione, 3β-hydroxy-2'-methyl-, 20-semicarbazone (8CI)](/CAS/20211123/GIF/16119-50-9.gif)
16119-50-9
0 suppliers
inquiry

14484-47-0
469 suppliers
$28.00/25mg