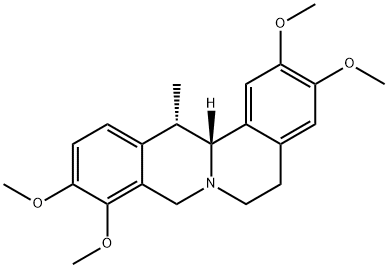
Corydaline synthesis
- Product Name:Corydaline
- CAS Number:518-69-4
- Molecular formula:C22H27NO4
- Molecular Weight:369.45
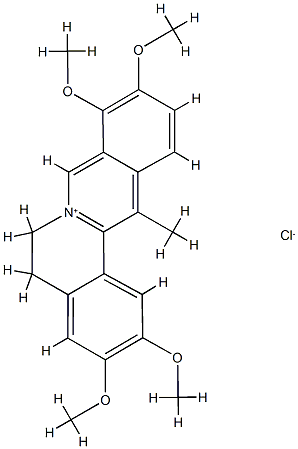
10605-03-5
36 suppliers
inquiry

518-69-4
185 suppliers
$23.00/1mg
Yield:518-69-4 91%
Reaction Conditions:
with 5,5’-bis(diphenylphosphino)-4,4’-bi-1,3-benzodioxole;di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]diiridium;hydrogen;glacial acetic acid;potassium bromide in dimethyl sulfoxide at 60; for 12 h;Autoclave;enantioselective reaction;
Steps:
5.Preparation of 13-MeTHPBs 4 via Ir-Catalyzed Asymmetric Hydrogenation
General procedure: A mixture of [Ir(COD)Cl]2(1.7 mg, 1.0 mol %) and (S)-SegPhons (3 mg, 2.0 mol %) was dissolved in a degassed mixed-solvent DMSO/AcOH (v/v = 9:1, 2 mL) in an argon atmosphere, and the resulting solution was allowed to be stirred at room temperature for 30 min. Then, 13-MePB3(100 mg, 0.25 mmol, 1.0 equiv.) and KBr (3 mg, 10 mol %) were added. The mixture was transferred to an autoclave, which was purged (3 × 20 atm) and charged with H2(80 atm); then, the reaction mixture was stirred at 60 °C for 12 h. The hydrogen gas was released slowly, and the solution was quenched with sat. Na2CO3aq. The resulting mixture was extracted with DCM (3 × 30 mL). The combined organic phase was dried with Na2SO4and evaporated in vacuum. The resulting residue was purified by silica gel chromatography (DCM) to givethe corresponding 13-MeTHPB alkaloids4.
References:
Chen, Wenchang;Yi, Xiaofen;Qu, Hongmin;Chen, Yu;Tang, Pei;Chen, Fener [Chinese Chemical Letters,2022,vol. 33,# 12,p. 5080 - 5083] Location in patent:supporting information
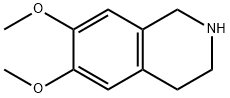
1745-07-9
115 suppliers
$8.00/250mg

518-69-4
185 suppliers
$23.00/1mg
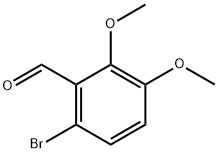
53811-50-0
22 suppliers
$172.00/50MG

518-69-4
185 suppliers
$23.00/1mg
29550-24-1
0 suppliers
inquiry

518-69-4
185 suppliers
$23.00/1mg
![6H-Dibenzo[a,g]quinolizine, 5,8,13,13a-tetrahydro-2,3,9,10-tetramethoxy-13-methyl-](/CAS/20210305/GIF/883833-45-2.gif)
883833-45-2
0 suppliers
inquiry

518-69-4
185 suppliers
$23.00/1mg