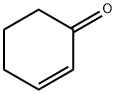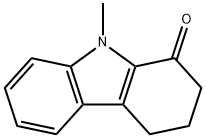
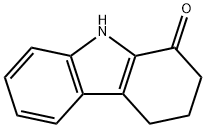
CHEMBRDG-BB 4013289 synthesis
- Product Name:CHEMBRDG-BB 4013289
- CAS Number:1485-19-4
- Molecular formula:C13H13NO
- Molecular Weight:199.25
3456-99-3
61 suppliers
$92.00/500mg

74-88-4
351 suppliers
$15.00/10g

1485-19-4
18 suppliers
$45.00/50mg
Yield:1485-19-4 100%
Reaction Conditions:
Stage #1: 1-oxo-2,3,4,9-tetrahydrocarbazolewith sodium hydride in acetone at 20; for 1 h;Inert atmosphere;
Stage #2: methyl iodide in acetone at 20; for 3 h;Inert atmosphere;
Steps:
10-methyl-2,4,5,10-tetrahydropyrazolo[3,4-a]carbazole (8)
General procedure: Under N2, to a solution of compound 3 (185mg, 1mmol) in 10mL of acetone was added NaH (200mg, 60% in coal oil, 7.5mmol) at room temperature. The mixture was stirred for 1h and then charged with MeI (255mg, 5mmol). After 3h, the mixture was quenched with water followed by extraction with CHCl3, dry with anhydrous Na2SO4 and evaporation in vacuo. The residue was purifiedby flash chromatography to give yellow solid in quantitive yield.
References:
Wu, Zhijie;Li, Ying;Cai, Yu;Yuan, Junying;Yuan, Chengye [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 17,p. 4903 - 4906] Location in patent:supporting information
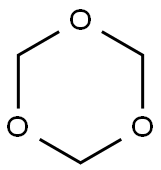
110-88-3
242 suppliers
$13.00/100g

3456-99-3
61 suppliers
$92.00/500mg

1485-19-4
18 suppliers
$45.00/50mg
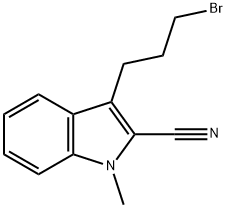
148237-24-5
0 suppliers
inquiry

1485-19-4
18 suppliers
$45.00/50mg
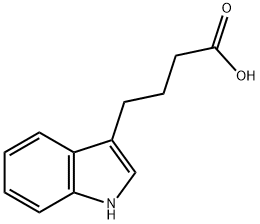
133-32-4
660 suppliers
$10.00/5g

1485-19-4
18 suppliers
$45.00/50mg