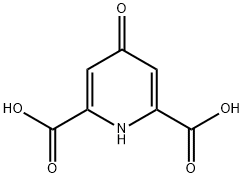
Chelidamic acid synthesis
- Product Name:Chelidamic acid
- CAS Number:138-60-3
- Molecular formula:C7H5NO5
- Molecular Weight:183.12
99-32-1
199 suppliers
$9.00/250mg

138-60-3
269 suppliers
$6.00/5g
Yield:138-60-3 98%
Reaction Conditions:
with ammonia in water at 20; for 48 h;
Steps:
13 4.1.2.12. Chelidamic acid (1,4-dihidro-4-oxo-2,6-pyridinedicarboxylic acid) (13)
Chelidamic acid was synthesized using an adapted literature protocol.33 In a typical experiment, 425 mL of NH3 (30% aqueous solution) was added dropwise at 0°C to 12 (41.8 g, 0.21 mol) over a period of 1 h. After the addition was complete, the resulting suspension was stirred at room temperature for 48 h. The excess of aqueous ammonia solution was removed under reduced pressure and the residue was refluxed for 15 min with 50 mL of water using charcoal. After filtration, the hot filtrate was collected and after return to room temperature acidified with HCl (37% aqueous solution) until pH=1. The resulting white solid was filtered off, washed several times with cold water and dried under vacuum for 16 h. The desired compound 13 was obtained as a white solid (42 g, 98%).
References:
Gracia, Stéphanie;Arrachart, Guilhem;Marie, Cécile;Chapron, Simon;Miguirditchian, Manuel;Pellet-Rostaing, Stéphane [Tetrahedron,2015,vol. 71,# 33,p. 5321 - 5336]