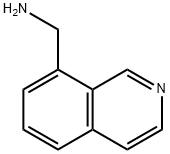
C-ISOQUINOLIN-8-YL-METHYLAMINE synthesis
- Product Name:C-ISOQUINOLIN-8-YL-METHYLAMINE
- CAS Number:362606-12-0
- Molecular formula:C10H10N2
- Molecular Weight:158.2
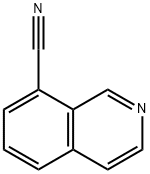
362606-11-9
66 suppliers
inquiry

362606-12-0
32 suppliers
inquiry
Yield:-
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 2 h;Inert atmosphere;
Steps:
Step B: Isoquinolin-8-ylmethanamine
[0206] A solution of isoquinoline-8-carbonitrile (210 mg, 1.36 mmol) in anhydrous THF (10 mL) was added dropwise to a slurry of LiAlH4 (103 mg, 2.72 mmol) in anhydrous THF (5 mL) at 0°C under nitrogen atmosphere. Upon completion of the addition, the cooling bath was removed and the mixture was stirred at ambient temperature for 2 hours. The reaction was quenched with Na2S04 10H2O (0.5 g), and then filtered. The filtrate was concentrated to give isoquinolin-8-ylmethanamine as an oil, which was used in the next step without further purification. LC/MS = 159 [M+l].
References:
WO2016/81290,2016,A1 Location in patent:Paragraph 0206
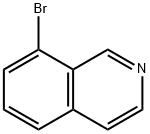
63927-22-0
258 suppliers
$7.00/250mg

362606-12-0
32 suppliers
inquiry