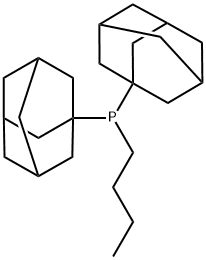
Butyldi-1-adamantylphosphine synthesis
- Product Name:Butyldi-1-adamantylphosphine
- CAS Number:321921-71-5
- Molecular formula:C24H39P
- Molecular Weight:358.54
31P NMR (C6D6)δ: 24.9. Mp 108-110°C. IR (KBr): 3425 (m, br), 2952 (s), 2847 (s), 2847 (s), 2675 (w), 1446 cm-1 (m). 1H NMR (250 MHz, C6D6): δ = 0.96 (3 H, t, 3JH, H = 7.3 Hz, CH3), 1.35-2.03 (36 H, m, adamantyl-30H, butyl-6H). 13C NMR (62 MHz, C6D6): δ = 41.3 (d, 2JC,P = 11.3 Hz, C-2), 37.4 (C-4), 36.1 (d, 1JC,P = 23.5 Hz, C-1), 33.9 (d, 1JC,P = 26.2 Hz, butyl-α -CH2), 29.1 (d, 3JC,P = 7.6 Hz, C-3), 24.9 (d, 2JC,P = 13.1 Hz, butyl-β -CH2), 17.1 (d, 3JC,P = 21.6 Hz, butyl-γ -CH2), 14.3 (butyl-CH3). MS (EI, 70 eV): m/z (%) = 358 (M+, 60), 135 (Ad+, 100).

109-65-9
551 suppliers
$10.00/10g
131211-27-3
151 suppliers
$36.00/200mg

321921-71-5
237 suppliers
$9.00/250mg
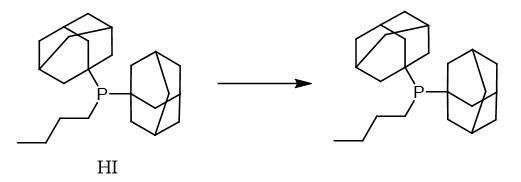
Yield:321921-71-5 4.6 g (13 mmol, 85%)
Reaction Conditions:
with n-butyllithium in toluene;
Steps:
1 Example 1
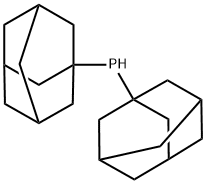
4.6 g (15 mmol) of di(1-adamantyl)phosphine were placed in 50 ml of di-n-butyl ether, and 20 ml of a 2.5 M solution of n-BuLi in toluene (50 mmol) were added. The mixture was refluxed for 1 h and cooled and 4.1 g (30 mmol) of 1-butyl bromide were added dropwise. The mixture was refluxed for 30 min, cooled and washed with saturated ammonium chloride solution (3*), the organic phase was separated off and dried over sodium sulfate and the solvent was distilled off under reduced pressure. Yield: 4.6 g (13 mmol, 85%) of di(1-adamantyl)-n-butylphosphine. The product can be recrystallized from di-n-butyl ether (m.p.: 102° C.). 31P{1H} NMR (162.0 MHz, C6D6, 297 K): δ=24.6
References:
US2004/68131,2004,A1
157282-19-4
78 suppliers
$93.00/250mg

321921-71-5
237 suppliers
$9.00/250mg

109-69-3
434 suppliers
$20.00/100ml

131211-27-3
151 suppliers
$36.00/200mg

321921-71-5
237 suppliers
$9.00/250mg

542-69-8
259 suppliers
$9.00/5g
131266-79-0
14 suppliers
$142.00/250mg

321921-71-5
237 suppliers
$9.00/250mg

542-69-8
259 suppliers
$9.00/5g

131211-27-3
151 suppliers
$36.00/200mg

321921-71-5
237 suppliers
$9.00/250mg