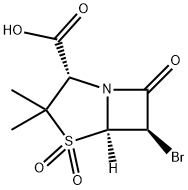
BROMOSULBACTAM synthesis
- Product Name:BROMOSULBACTAM
- CAS Number:75527-87-6
- Molecular formula:C8H10BrNO5S
- Molecular Weight:312.14
![[2S-(2alpha,5alpha,6alpha)]-6-bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid](/CAS/GIF/24138-28-1.gif)
24138-28-1
29 suppliers
inquiry

75527-87-6
58 suppliers
$460.00/10mg
Yield:-
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in hexane;chloroform;ethyl acetate
Steps:
(2S,5R,6S)-6α-Bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]-heptane-2-carboxylic acid, S,S-dioxide.
(2S,5R,6S)-6α-Bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]-heptane-2-carboxylic acid, S,S-dioxide. To a cold (0-5° C.) solution of m-chloroperbenzoic acid (20.00 g) in ethyl acetate (200 ml) is added (2S,5R,6S)-6-bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (5.6 g, 20 mM) in ethyl acetate (30 ml). After stirring overnight at 26° C., the reaction mixture is diluted with ethyl acetate (300 ml) and extracted with saturated sodium bicarbonate solution (2*150 ml). The combined aqueous extracts are acidified to pH 2 with concentrated hydrochloric acid and extracted quickly with ethyl acetate (3*200 ml). The combined organic extracts are dried over sodium sulfate and concentrated under reduced pressure and elevated temperature to afford a white solid. This material is a mixture of the desired product and m-chlorobenzoic acid. Trituration (3*) with hexane (1 liter), yields a white solid (5.7 g) free of almost all m-chlorobenzoic acid. An analytical sample is prepared as follows. Approximately 1 g of product is dissolved in chloroform/hexane and brought to the cloud point. After one hour, the filtrate is decanted from oil which has deposited on the flask. Upon standing for about 16 hours the filtrate gives crystals, which are recrystallized from hot benzene to yield the title compound, melting point 139°-140° C.; IR (KBr) 1793, 1780 (sh), 1755 cm-1; NMR (acetone-d6) 1.50 (s, 3H), 1.60 (s, 3H), 4.48 (s, 1H), 5.16 (d, 1H, J=2 Hz), 5.40 (d, 1H, J=2 Hz). Anal. Calc'd. for C8 H10 BrNO5 S: C, 30.78; H, 3.23; N, 4.48; S, 10.27; Br, 25.60. Found C, 30.98; H, 3.11, N, 4.62; S, 10.32; Br, 25.52.
References:
E. R. Squibb & Sons, Inc. US4203993, 1980, A
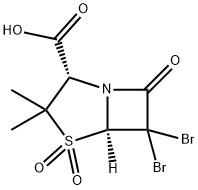
76646-91-8
65 suppliers
$165.00/25mg
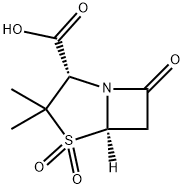
68373-14-8
355 suppliers
$5.00/100mg

75527-87-6
58 suppliers
$460.00/10mg
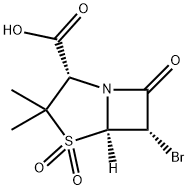
75527-88-7
17 suppliers
inquiry